- Therapeutic Solutions
-
-
-
Therapeutic Solution
Ebola
COVID-19
Oncology
-
-
-
- Medical Animations
- Commercial Services
- Company Infrastructure
- People
- News
- Invest
- Contact
Oya1 originally was first discovered as broad microbicide and an Ebola antiviral in collaboration with NIAID. This work was recently published (Bennett et al., (2021) ‘A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures’ Viruses Viruses 13 https://doi.org/10.3390) and is patent protected. The published data show that Oya1 is markedly more effective than remdesivir but has significant virus stopping capability alone or in combination with remdesivir. Clinical trials showed that Mab therapy had greatest therapeutic value in treating Ebola. Vaccination strategies for Ebola and remdesivir were minimally effective for people infected with Ebola who already have symptoms. The unmet need is that protection through immunization takes 21 days but the virus kills in 14 days. When an Ebola vaccine becomes available, it may prevent the spread of Ebola to those who are immunized but it will not prevent transmission or death of persons within the disease epicenter who are not immune. Mab production and distribution are costly and require special infrastructure. OyaGen will address the unmet need for highly active antiviral therapies that are low in cost of production and distribution.
Oya1 has an inexpensive five step synthesis, has a long shelf-life, and does not require special considerations for storage or shipment. Pre-IND discussions with the FDA have confirmed studies that will gate the entry of Oya1 for human Phase I clinical trials. The company seeks to partner or license Oya1 or obtain a tranched capital raise enabling OyaGen to complete remaining preclinical studies required by the FDA for approval of an IND and Phase I clinical safety and pharmacokinetic studies.
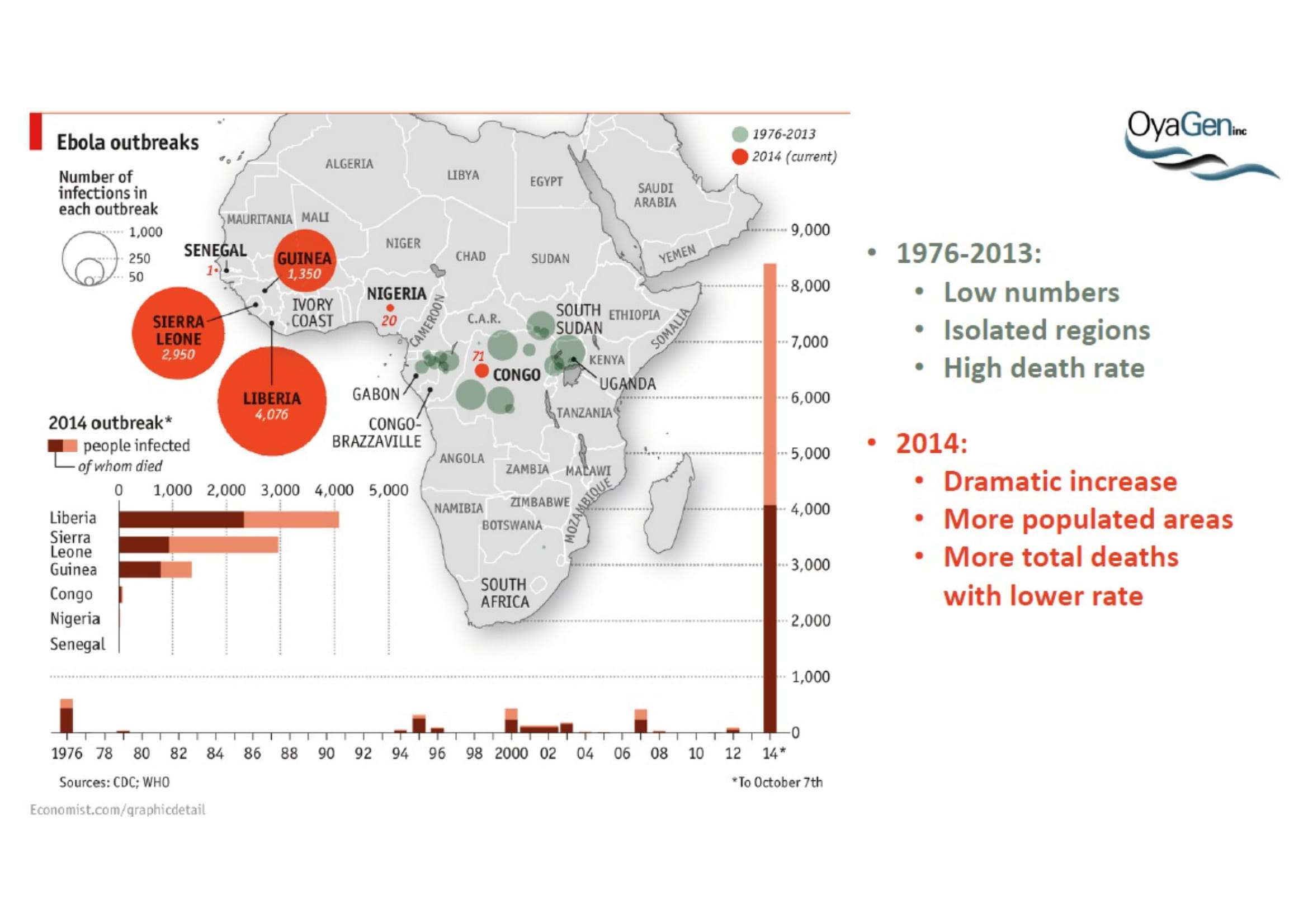
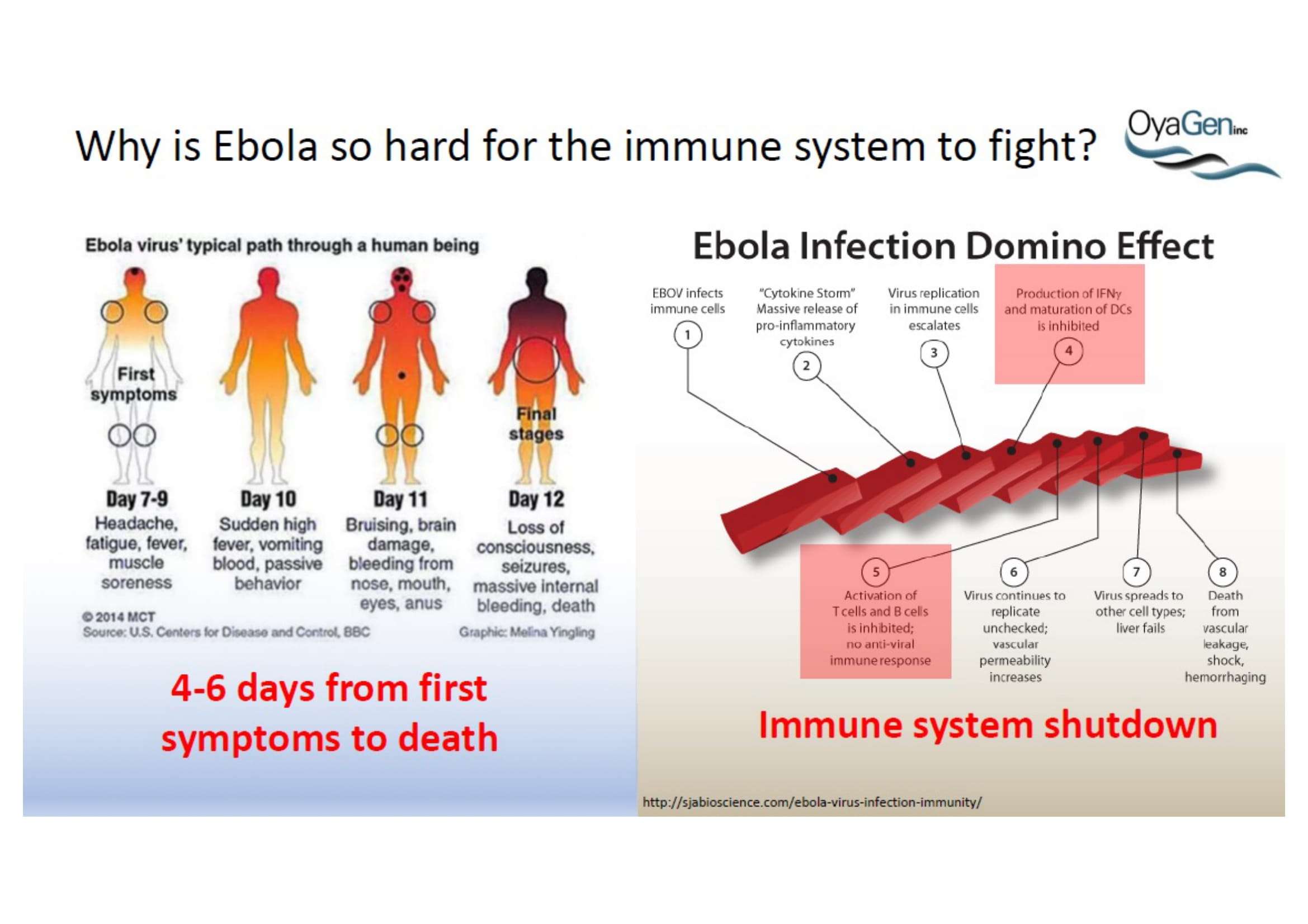
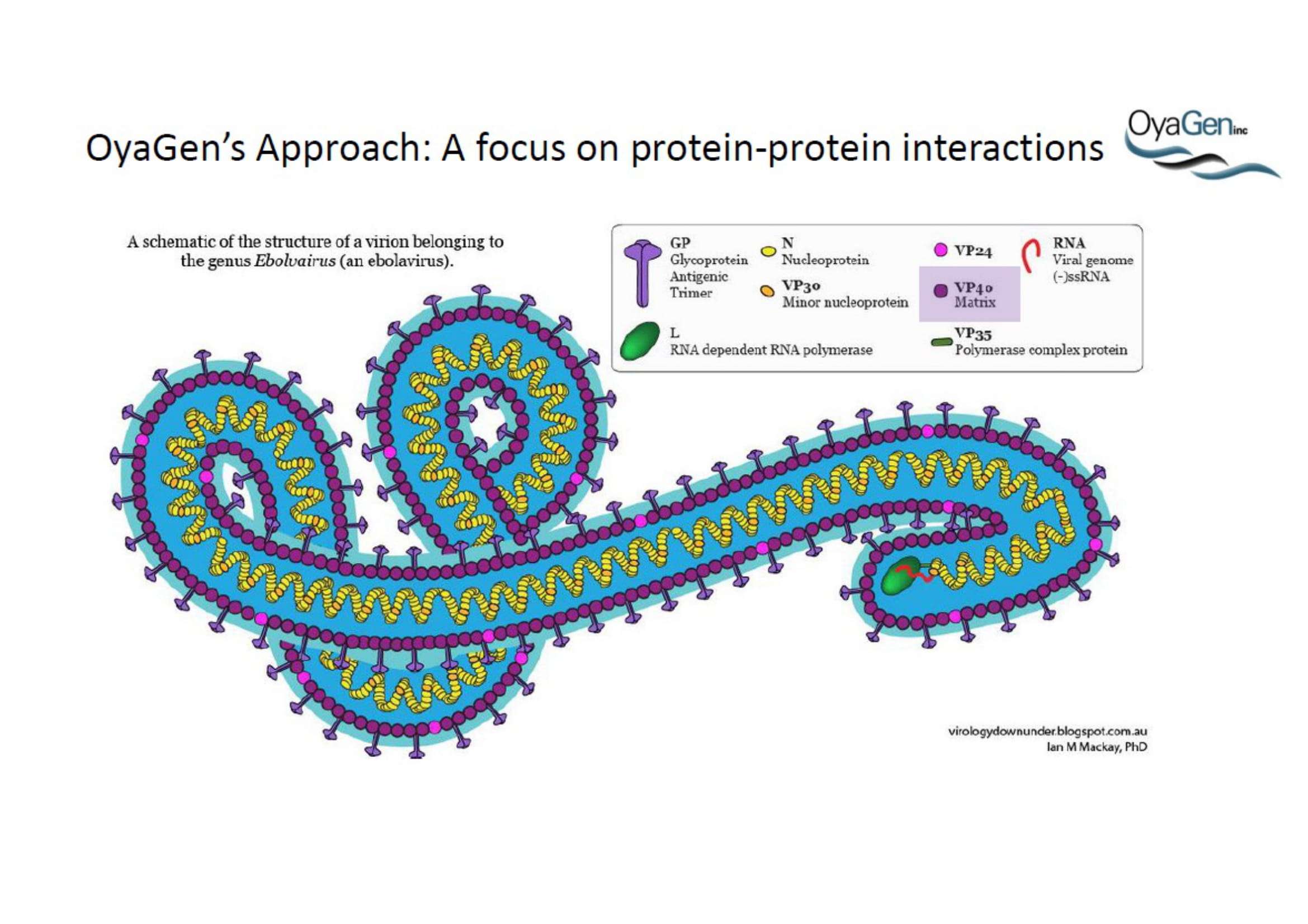
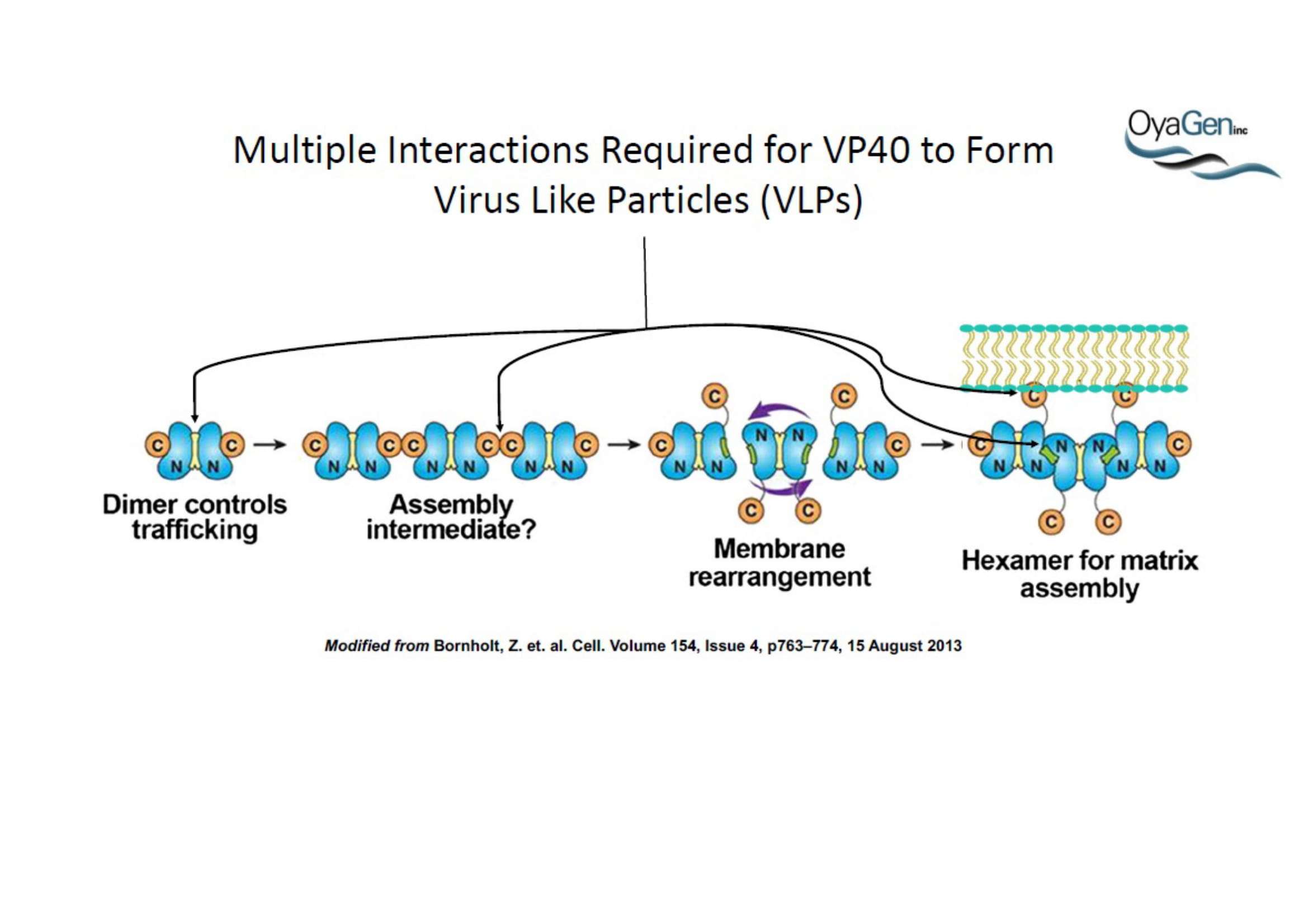
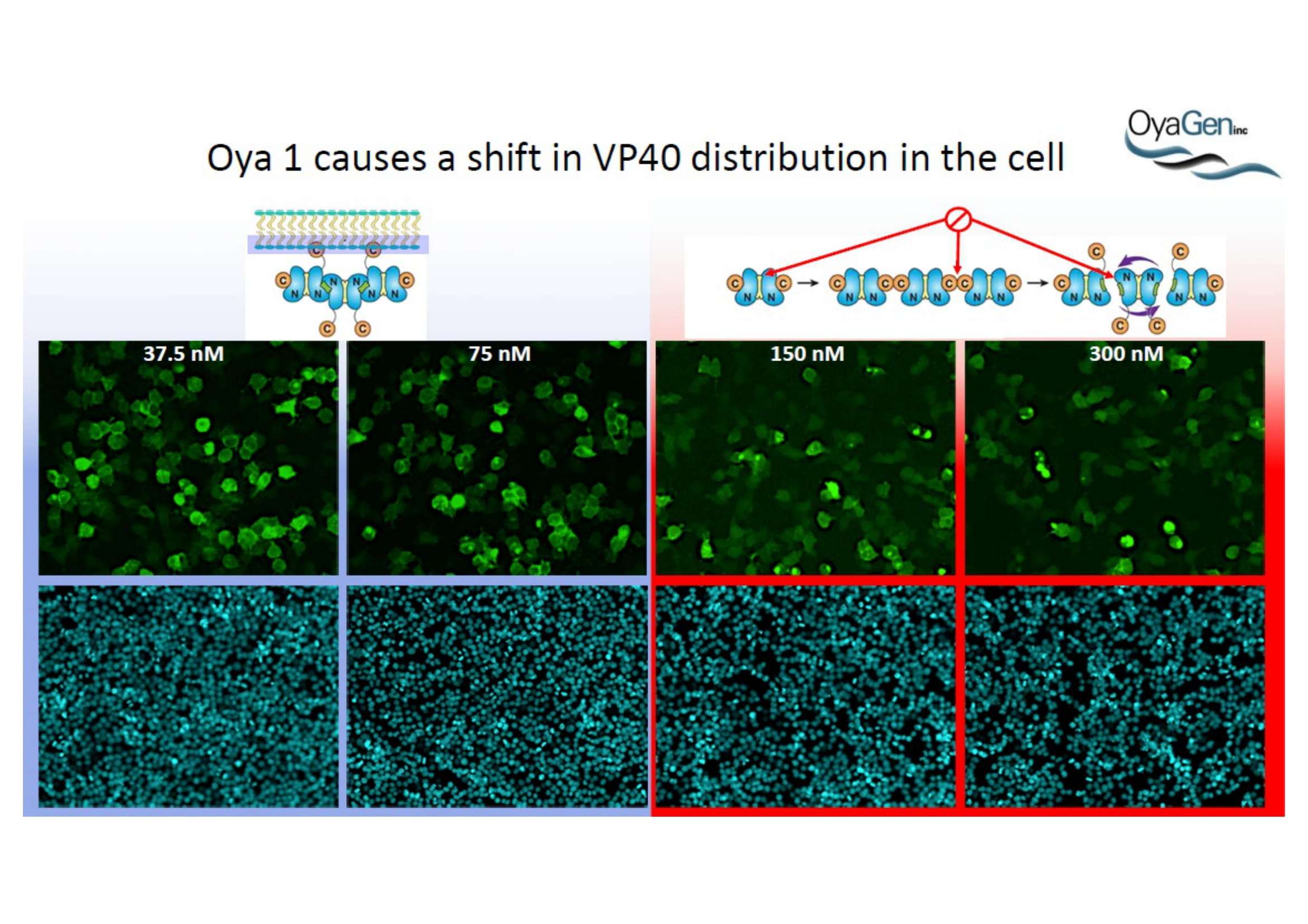
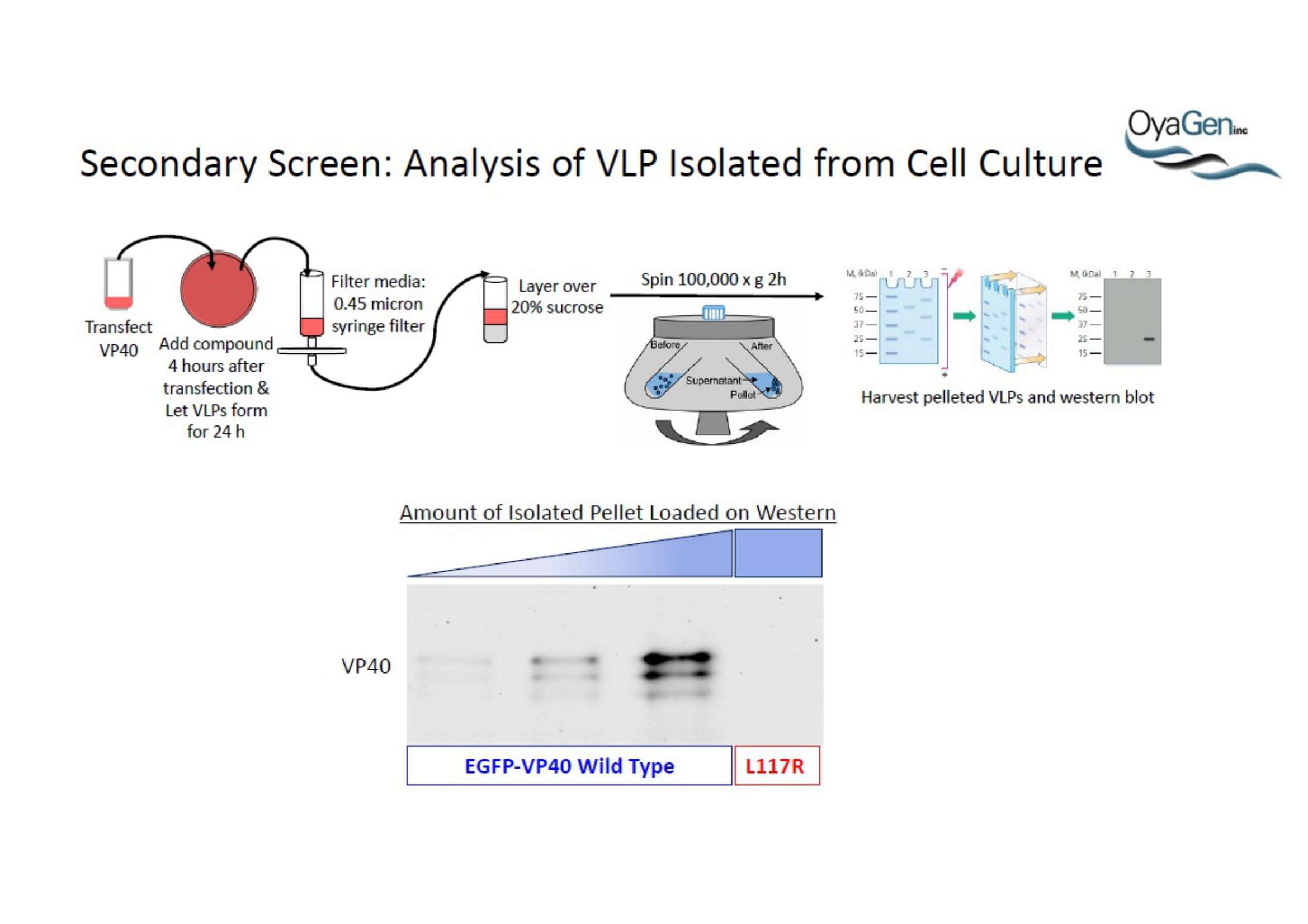
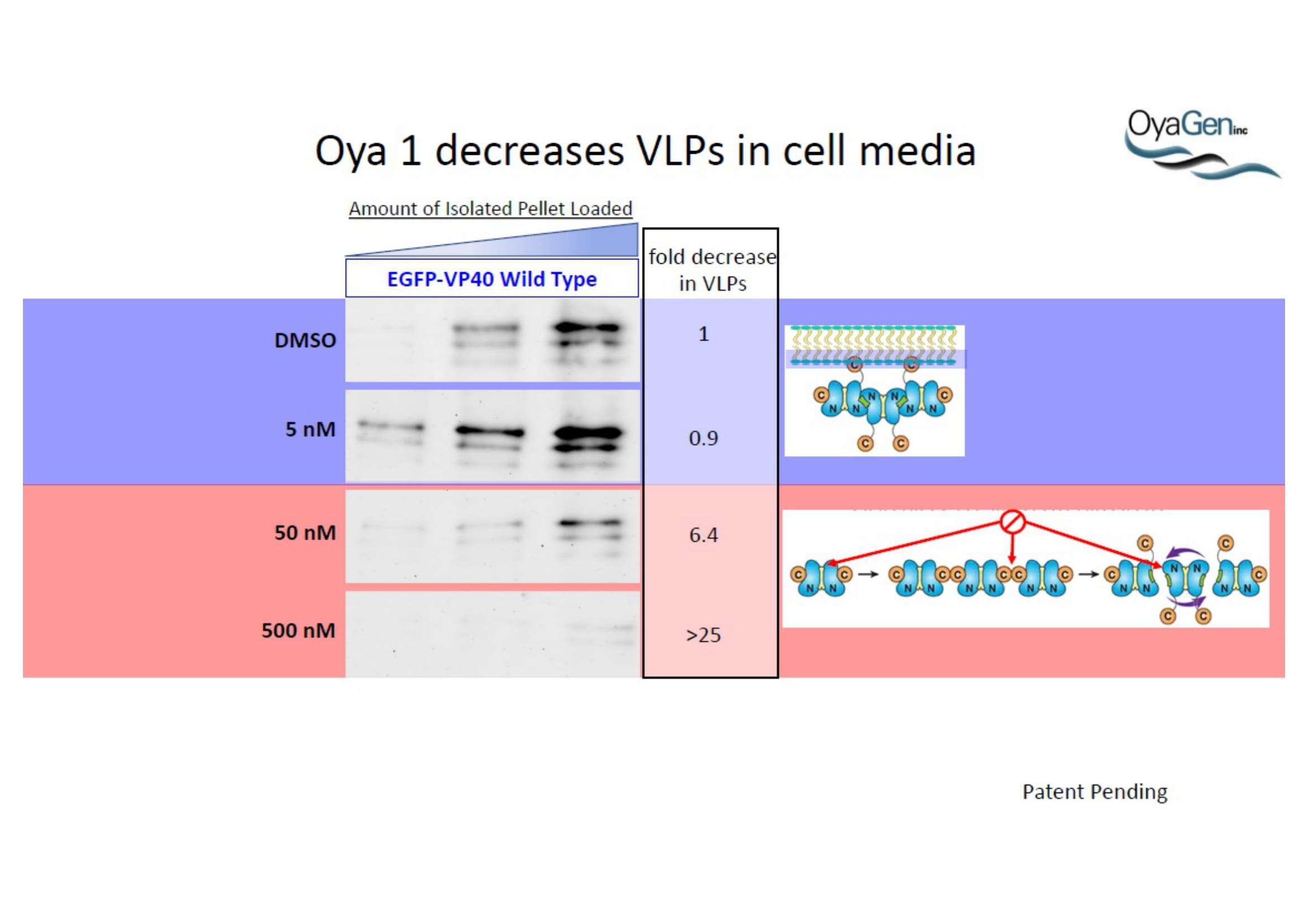

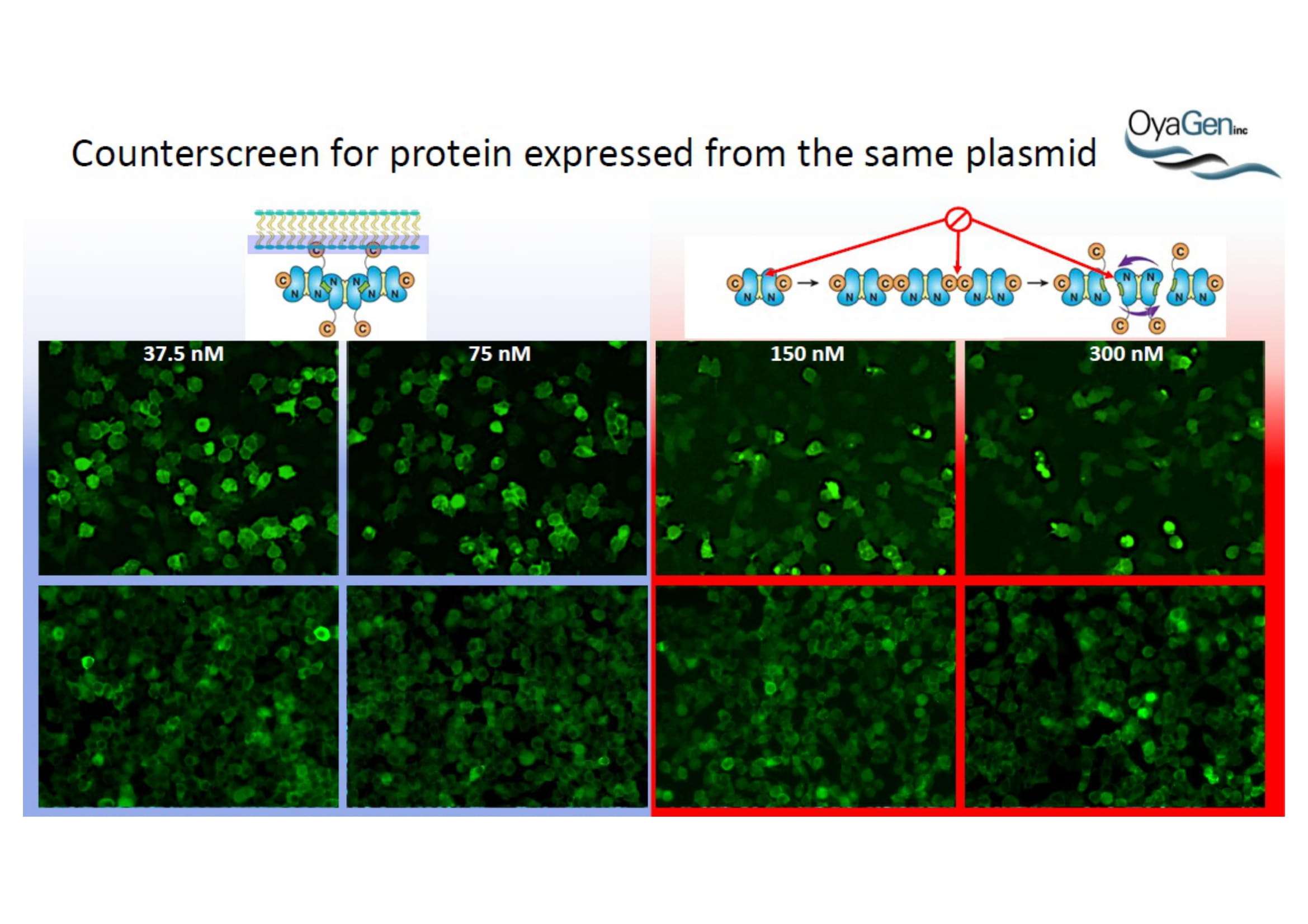
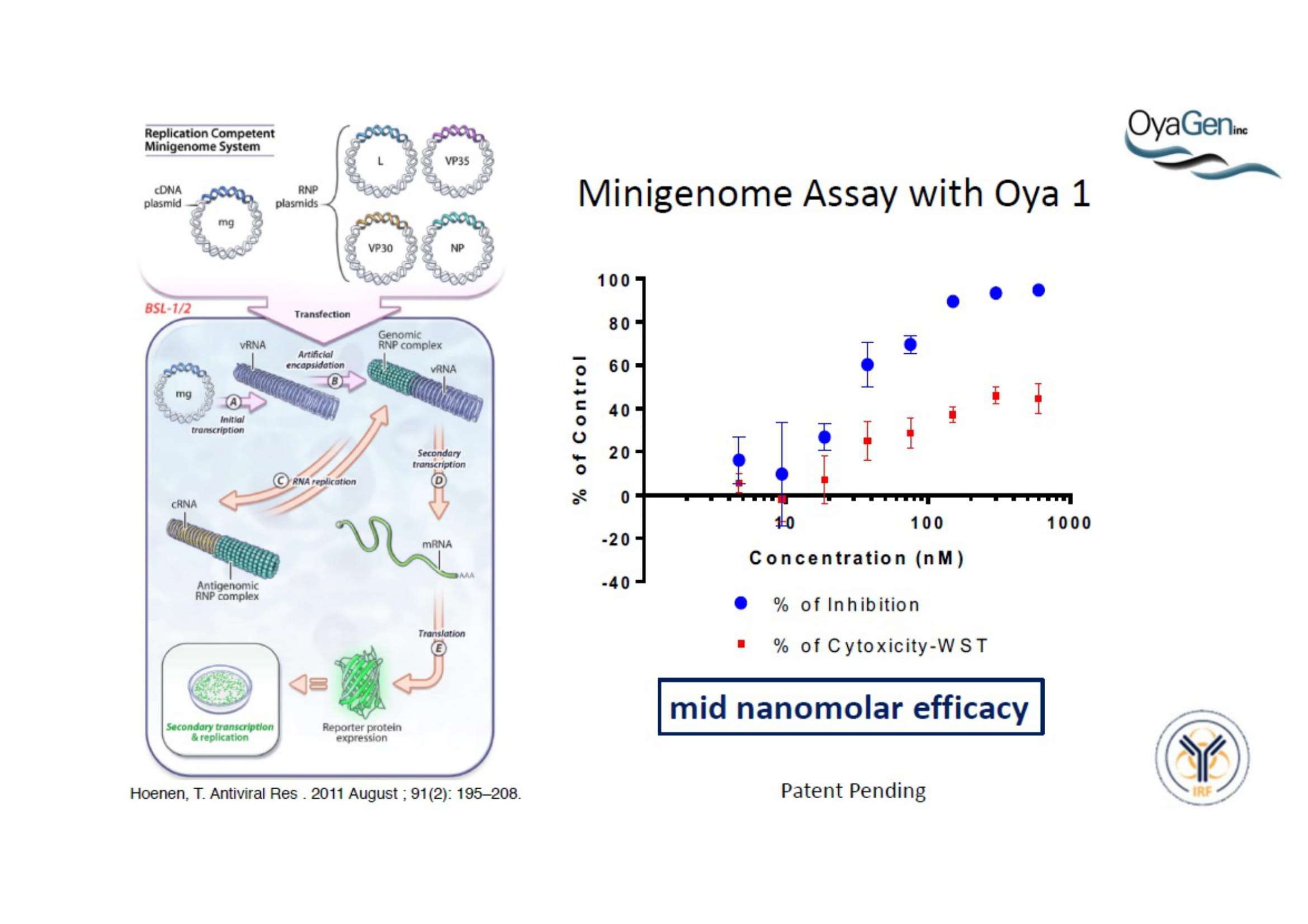
OyaGen, Inc has developed Oya1, a highly potent and cost effective antiviral therapeutic for Ebola with intended use in the treatment for patients with an active Ebola infection. Oya1 will address the unmet need for an effective countermeasure to fill the gaps where vaccines do not have an immediate benefit for patients who are already infected or are immune-compromised. Combined treatments of Oya1 and remdesivir or monoclonal antibodies are anticipated to markedly enhance therapeutic efficacy while mitigating the emergence of drug resistant Ebola.
NIH/NIAID has shown that Oya1 blocks the virus from making copies of itself. It does so at low and high virus levels and even when added before or after the virus enters cells.
NIH/NCI has shown that Oya1 can be safely administered across a range of doses and dose intervals when tested in humans enrolled in prior cancer clinical trials.
There is an immediate and long-term demand for Oya1 regardless of the availability of vaccines or monoclonal antibody (Mab) therapies. Vaccines are not therapeutic; they alone are not effective in reducing active disease in people who already have Ebola because of the time it takes to mount an immune response. All vaccines could be given to ‘sick’ people in combination with a fast acting therapeutics such as Oya1 that do not depend on an immune response.
While Mab treatments are intended for treating infected patients, like vaccines, they may only be effective for the current strain of Ebola (e.g. Flu vaccines). Mab require specialized lab resources for their production. They are biologics that are very expensive to produce and may require special storage conditions.

© 2025, OyaGen, Inc.
SMARTSite by Site Hub