- Therapeutic Solutions
-
-
-
Therapeutic Solution
Ebola
COVID-19
Oncology
-
-
-
- Medical Animations
- Commercial Services
- Company Infrastructure
- People
- News
- Invest
- Contact
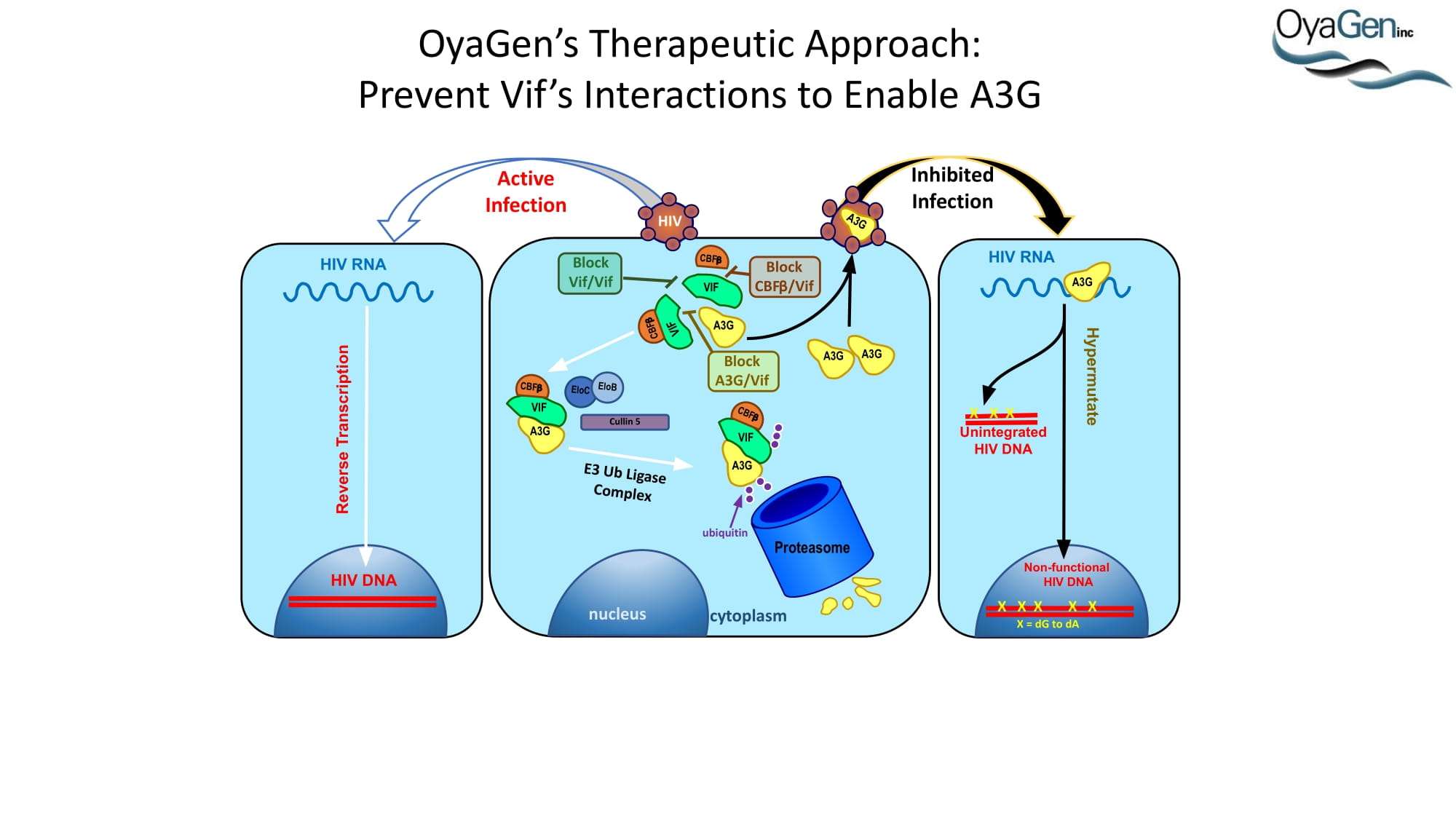
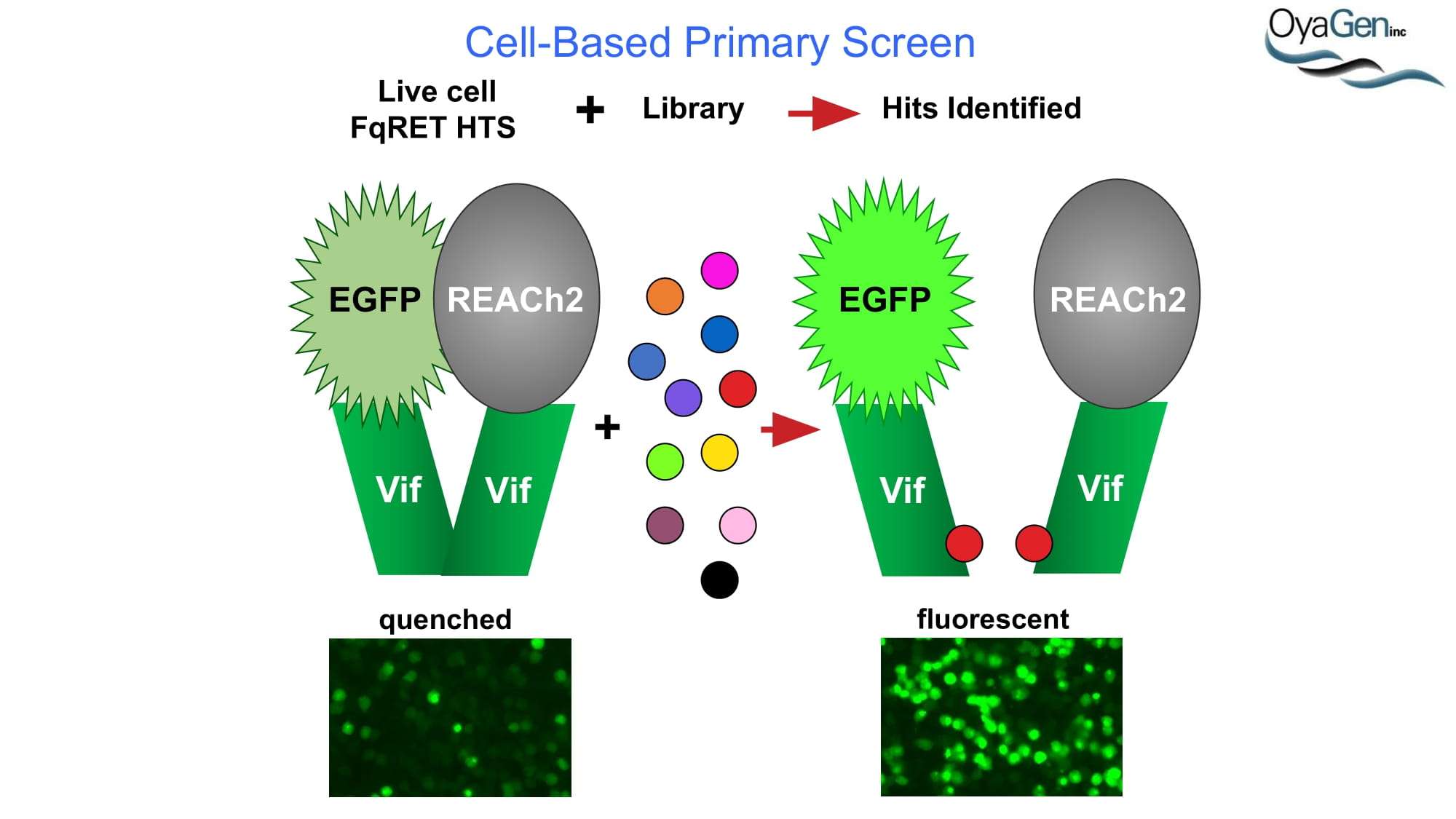
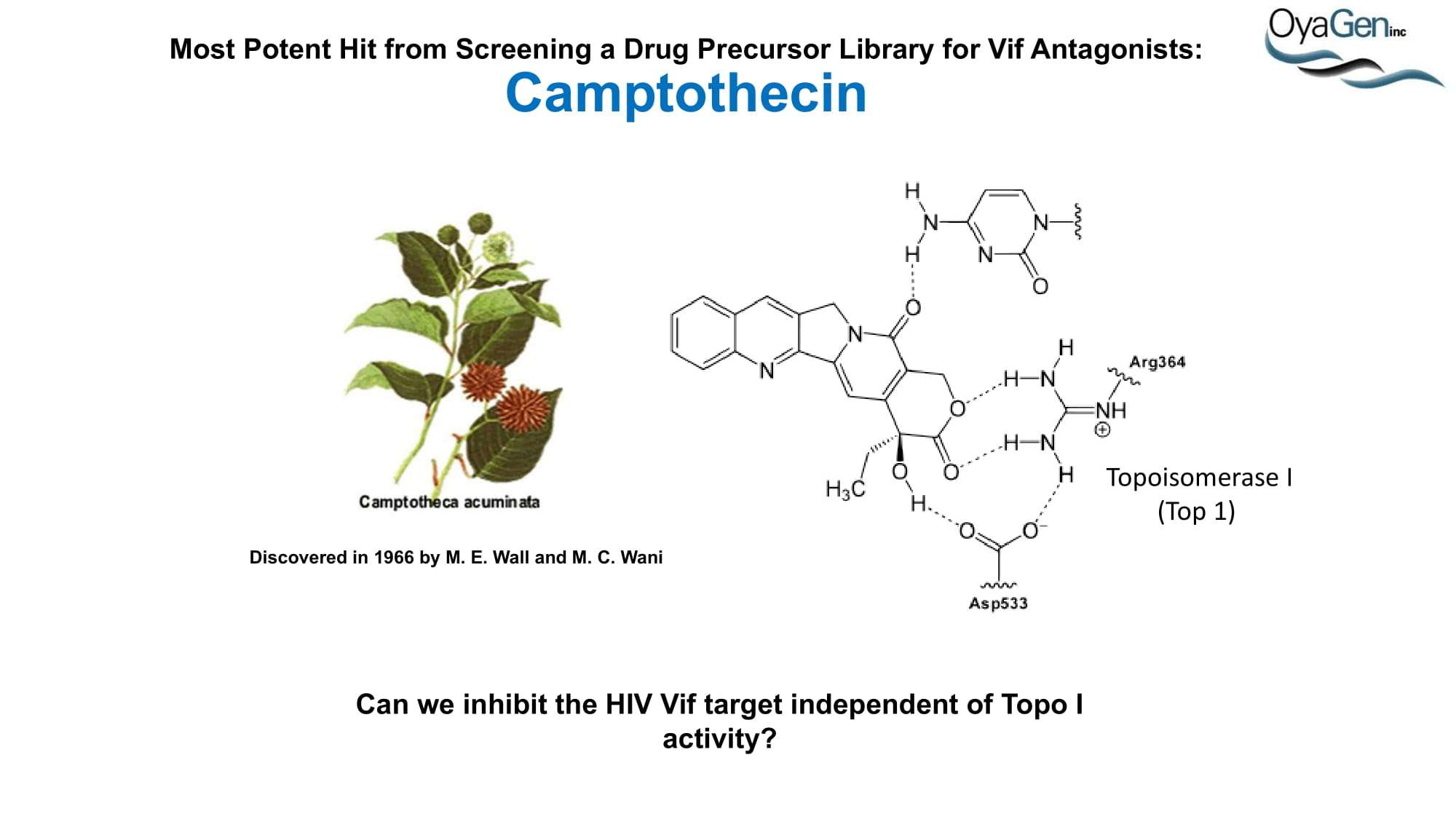
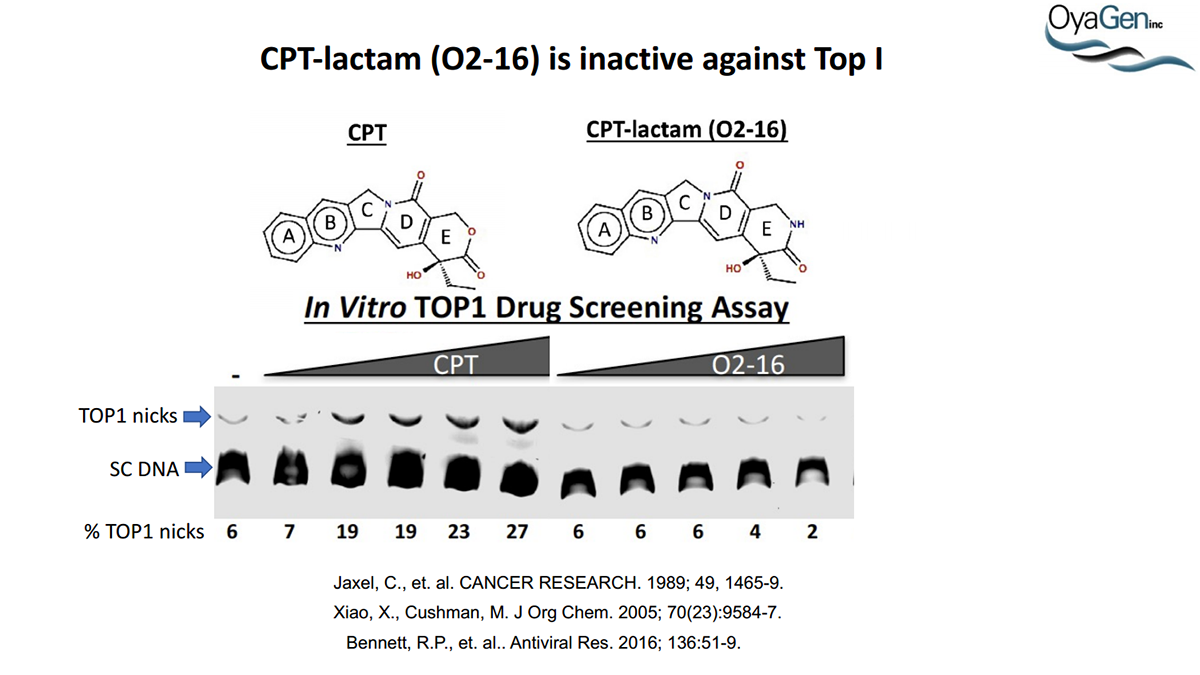
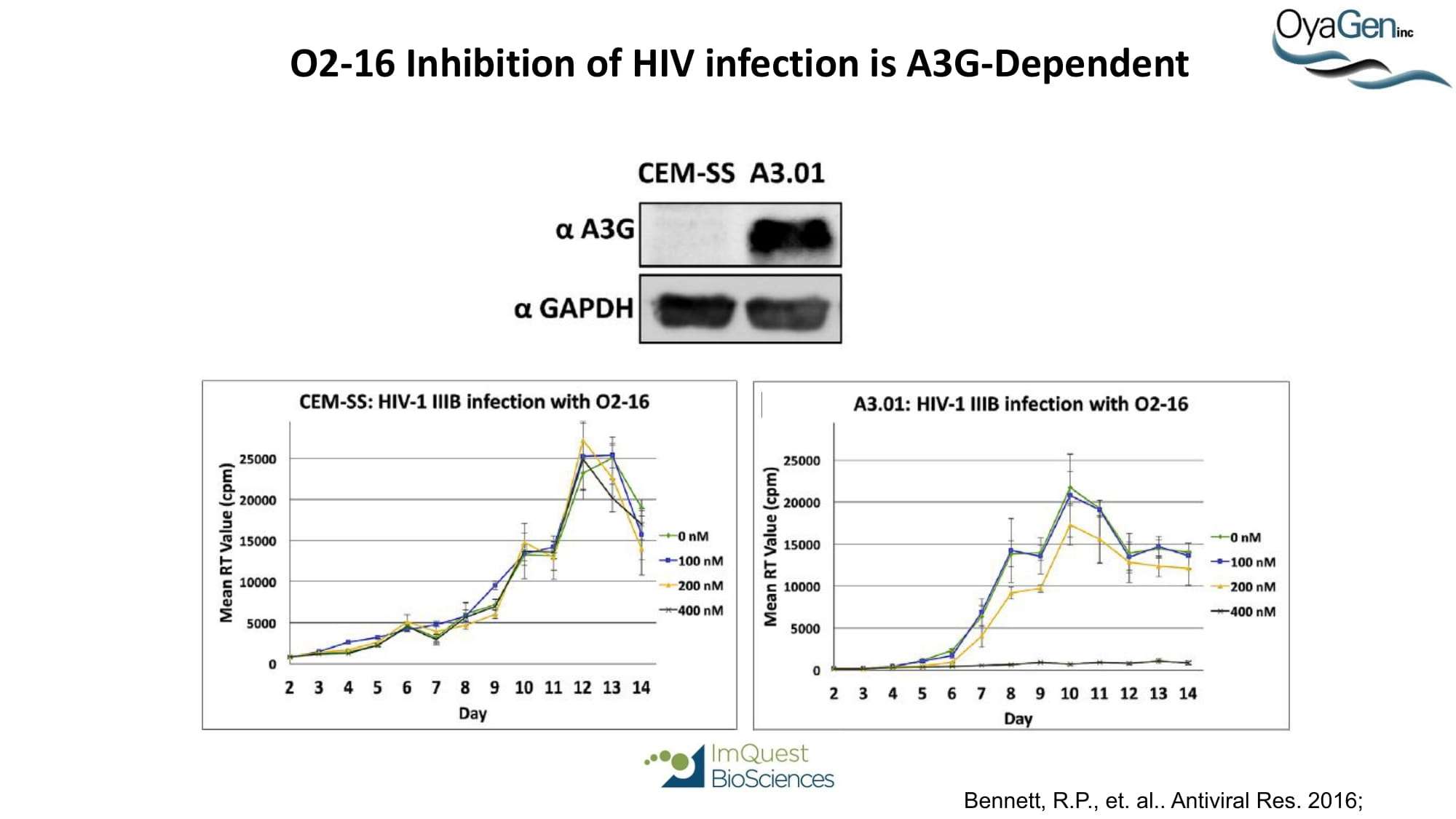
OyaGen is on track to start IND-enabling studies and engage the FDA in preIND discussions for our lead Vif antagonist, a patent-protected, first-in-class antagonist of the HIV-1 Vif protein that protects the APOBEC innate immune system and blocks HIV replication through gene editing. Formulation for oral dosing is the Company’s top priority. OyaGen has developed O2-16 through grants and contract research with NIAID (Bennett, R.P., et.al. Antiviral Research 136:51-9 PMC5125868) and has follow on antagonists in development along the Vif APOBEC3G pathway. O2-16 not only has therapeutic potential but holds the potential to reduce viral reservoir formation as part of a strategy for a cure and prevention (Trends in Molecular Medicine Published online March 30, 2018 PMID:29609878. Review).

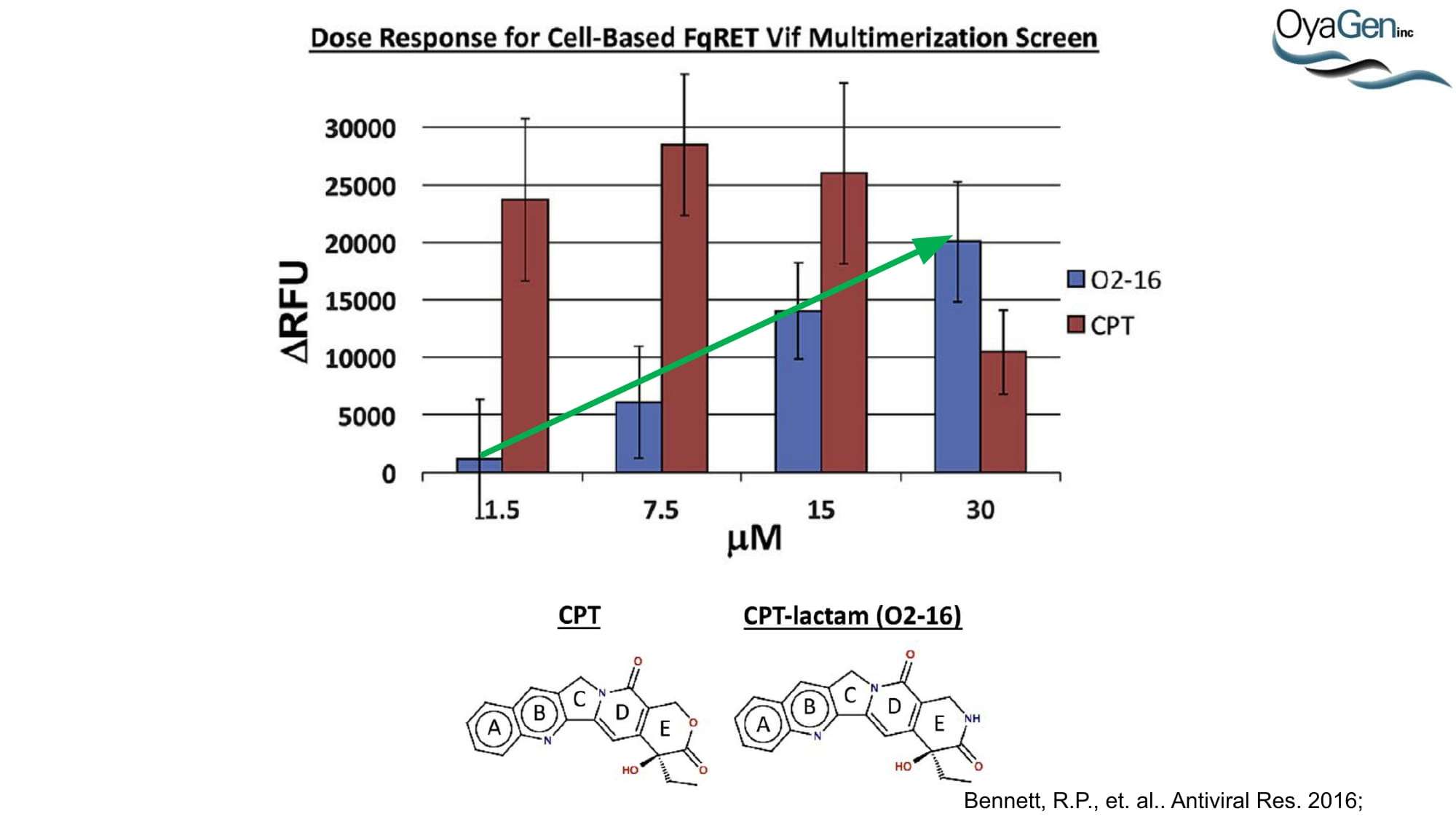
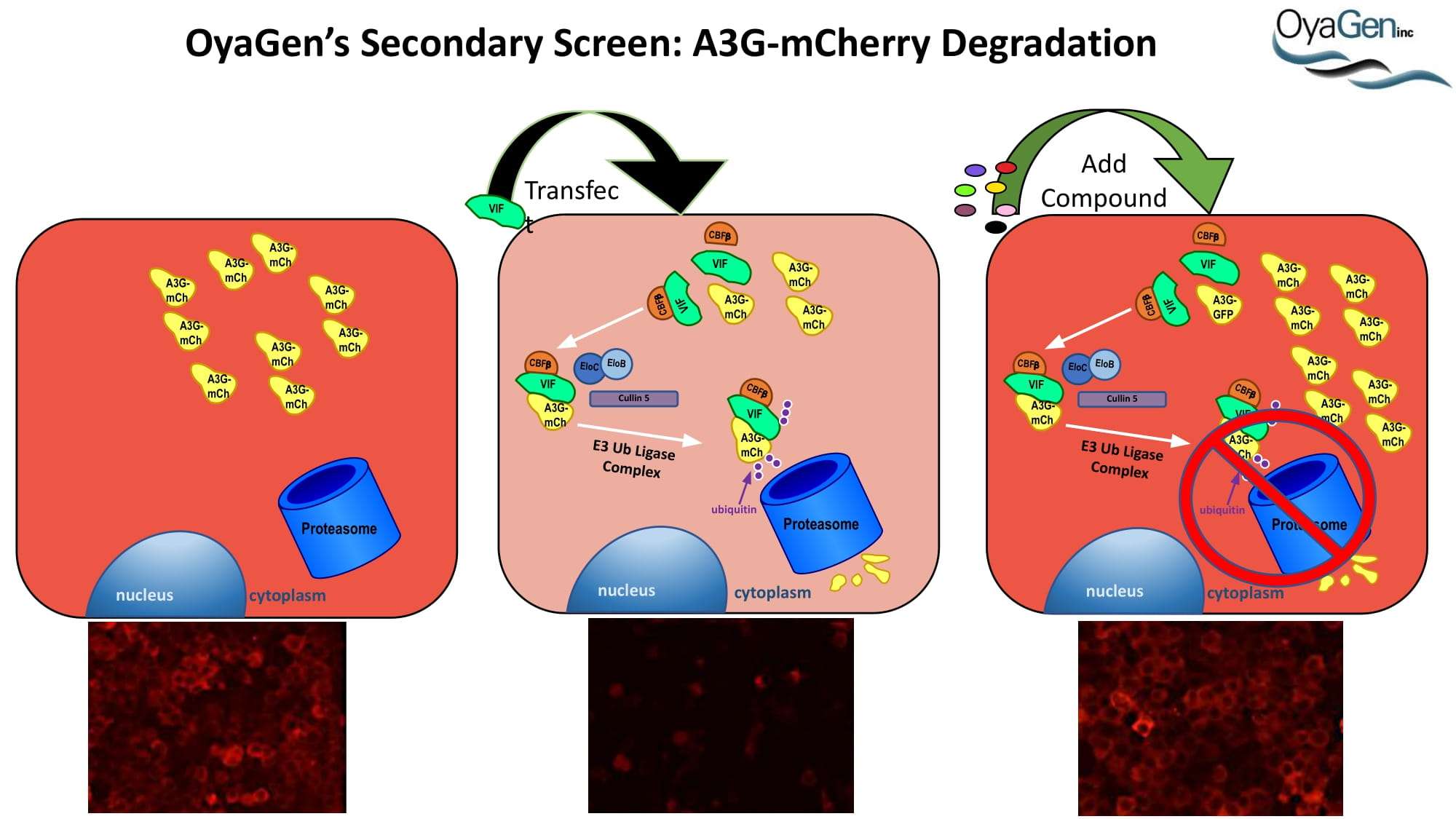
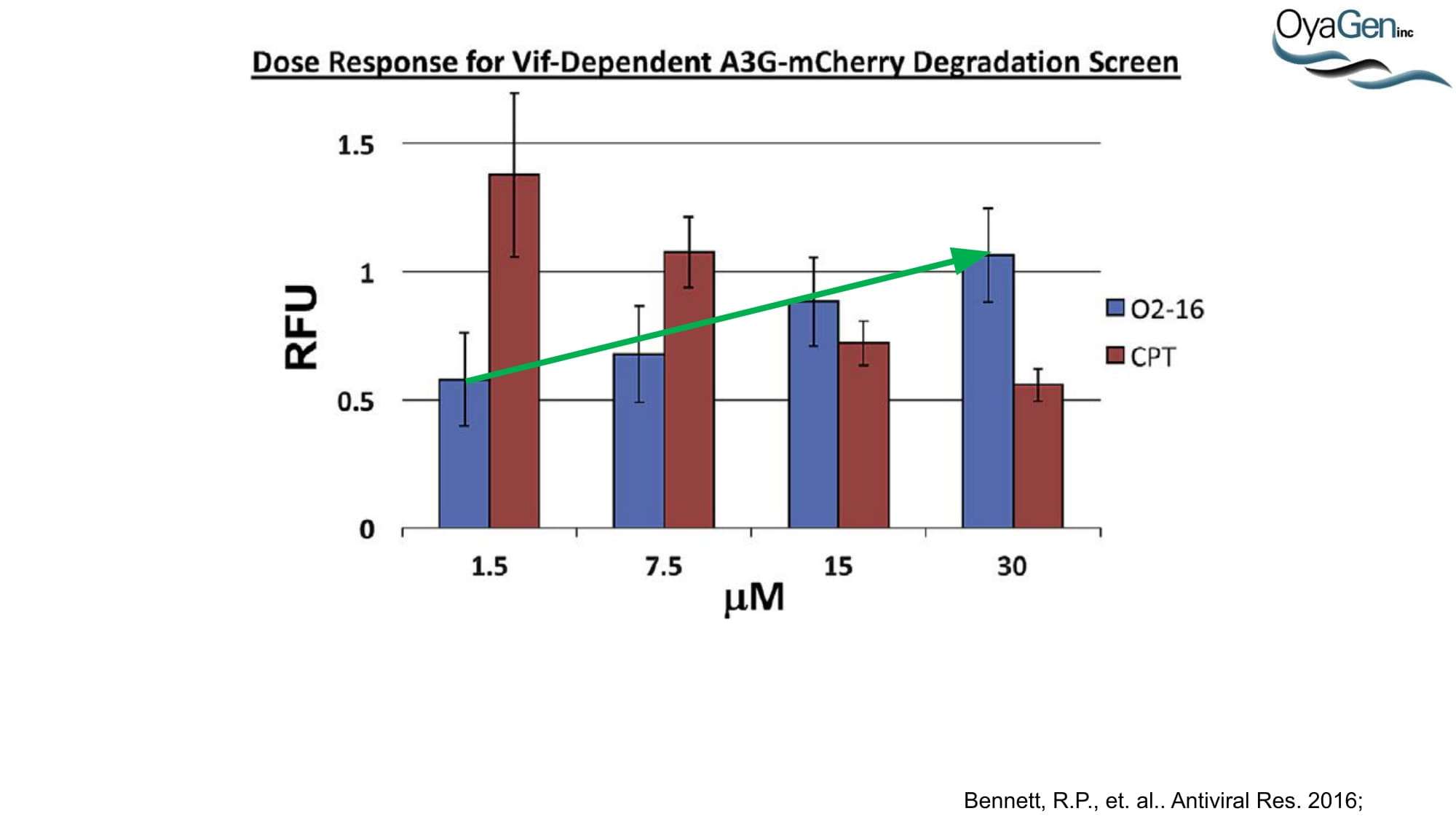
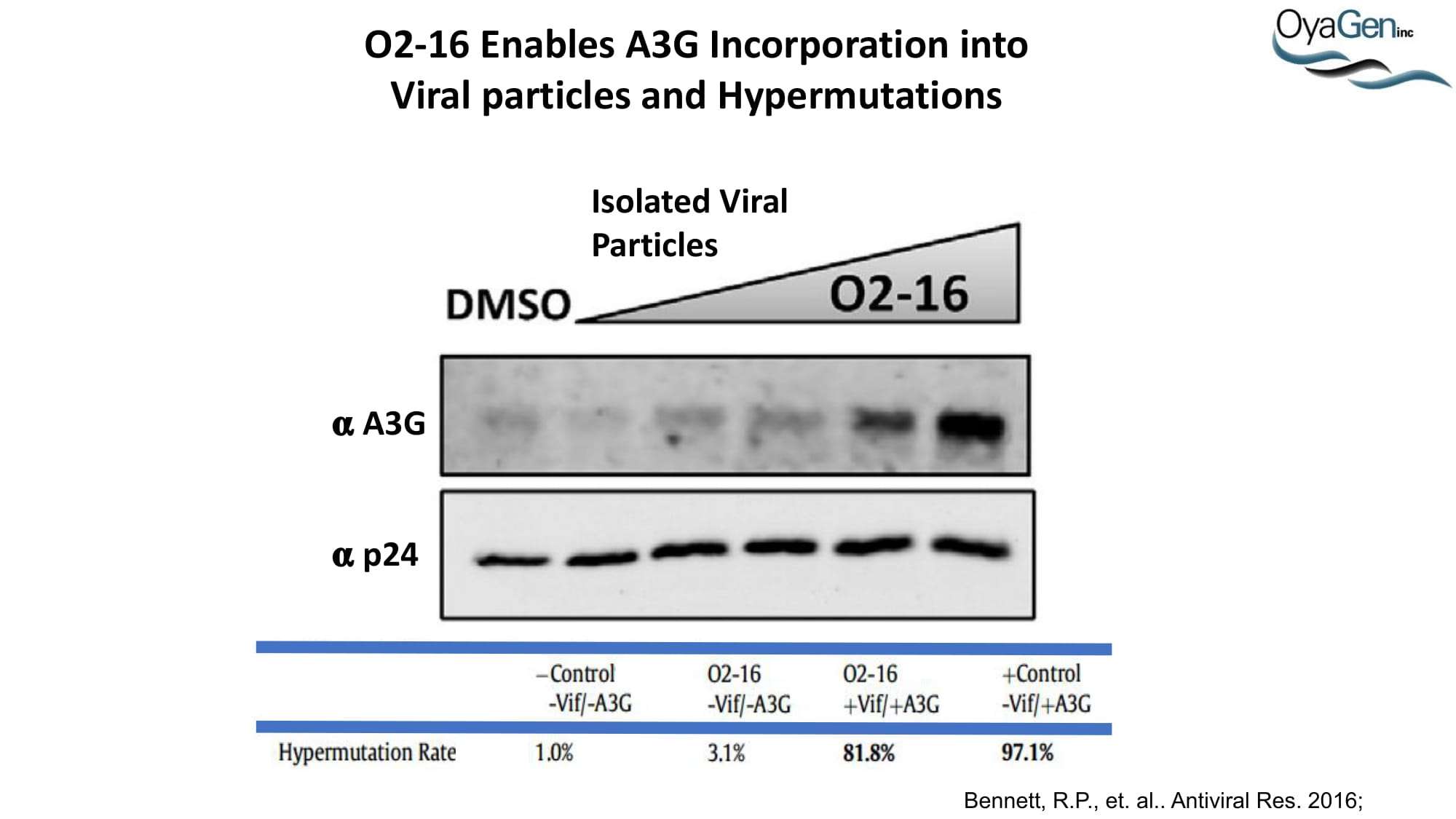
Vif antagonist will have significant market pentation because there remains a significant health care unmet need due to new infections and 30+ million HIV/AIDS patents worldwide. The drug market is expected to continue to show sustained growth despite advances in controlling the progress of the disease (Cowen & Company). The incidence of HIV continues to grow at a double-digit rate in developed markets according to the U.S. Center for Disease Control. Patient treatment remains low. Approximately 30% of US citizens infected with HIV are unaware they are HIV positive While treated patients have longer survival, the failure rates for current frontline therapies are 10% and ultimately the virus’s ability to mutate continues to demand new drugs. Each first-in-class HIV drug has achieved blockbuster annual sales (Cowen & Co.). In recent years the Pharma pipeline for new class of HIV therapeutics have dried up largely due to reduction in R&D expenditure. OyaGen preclinical development for a first-in-class treatment is unique in the global effort and has significant potential for treatment, prevention and cure.
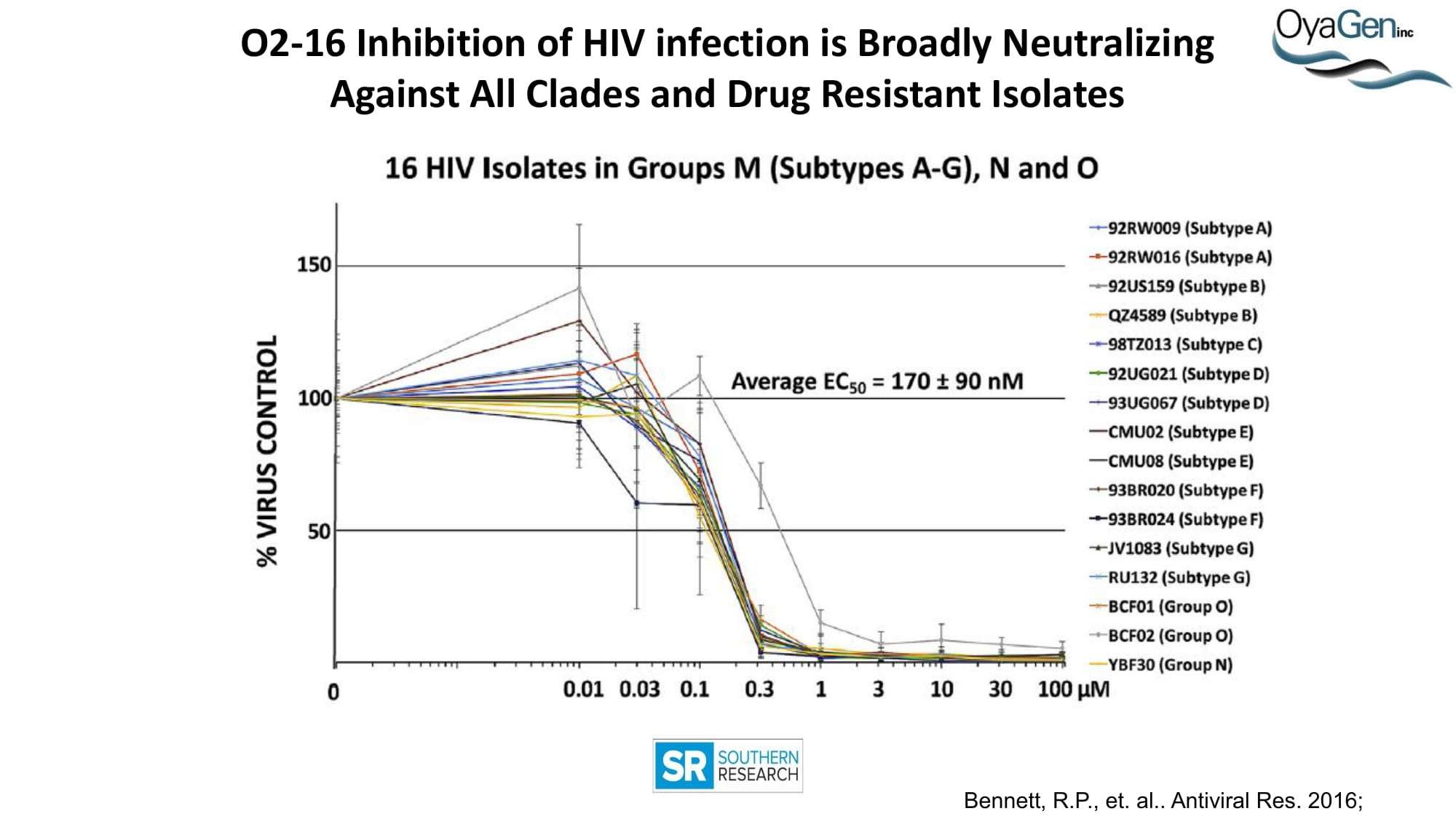
OyaGen has in late-stage preclinical development a unique therapeutic lead for HIV/AIDS that is 14-16 months from a pre-IND meeting with the FDA. Despite several FDA approved treatments, HIV remains a significant health care issue with >60 million people worldwide affected. The emergence of drug resistant HIV strains, enhanced by inconsistent access to a continuum of care continues to promote the spread HIV globally. The incidence of HIV continues to grow at a double-digit rate according to the U.S. Center for Disease Control. Approximately 30% of US citizens infected with HIV are unaware they are HIV positive. While treated patients have longer survival, the failure rate for current frontline therapies is 10% and ultimately the virus’s ability to mutate continues to demand new drugs. Consequently, the international drug market is expected to continue to show sustained growth despite advances in controlling the virus for those patients with access to treatment (Cowen & Co.). Our current preclinical efforts have shown our leads to be broadly neutralizing of all strains of HIV and additive in efficacy with all commercial highly active anti-HIV therapeutics. We have developed the chemical path with CROs for producing pure compounds and are developing oral formulations that will enable sustained blood levels for once-a-day dosing.
Each first-in-class HIV drug has achieved blockbuster annual sales (Cowen & Co.). In recent years the Pharma pipeline for new classes of HIV therapeutics has dried up largely due to reduction in R&D expenditure. PimStrategus Professional Valuation Services research suggested that while OyaGen’s lead compound has great potential in HIV therapeutics and will be effective against new strains of HIV that may be resistant to current treatments, it has high potential for a block-buster status in the areas of HIV prevention (PrEP) and HIV cure. In that regard, OyaGen has initiated in vitro studies in reservoir cells assessing the potency to neutralize viremia associated with reservoir cell reactivation as proof-of-concept that OyaGen’s lead compound could be part of a functional cure strategy.
HIV remains a significant health care issue with 33 million people worldwide affected, 1.5 million in the USA and EU. The market is expected to continue to show sustained growth despite advances in controlling the progress of the disease (Cowen & Company). The incidence of HIV continues to grow at a double digit rate in developed markets according to the U.S. Center for Disease Control. Patient treatment remains low.
30% of USA patients are unaware they are HIV positive and only one third of HIV positive USA patients receive treatment.
While treated patients have longer survival, the failure rates for current frontline therapies are 10% and ultimately the virus ability to mutate continues to demand new drugs.
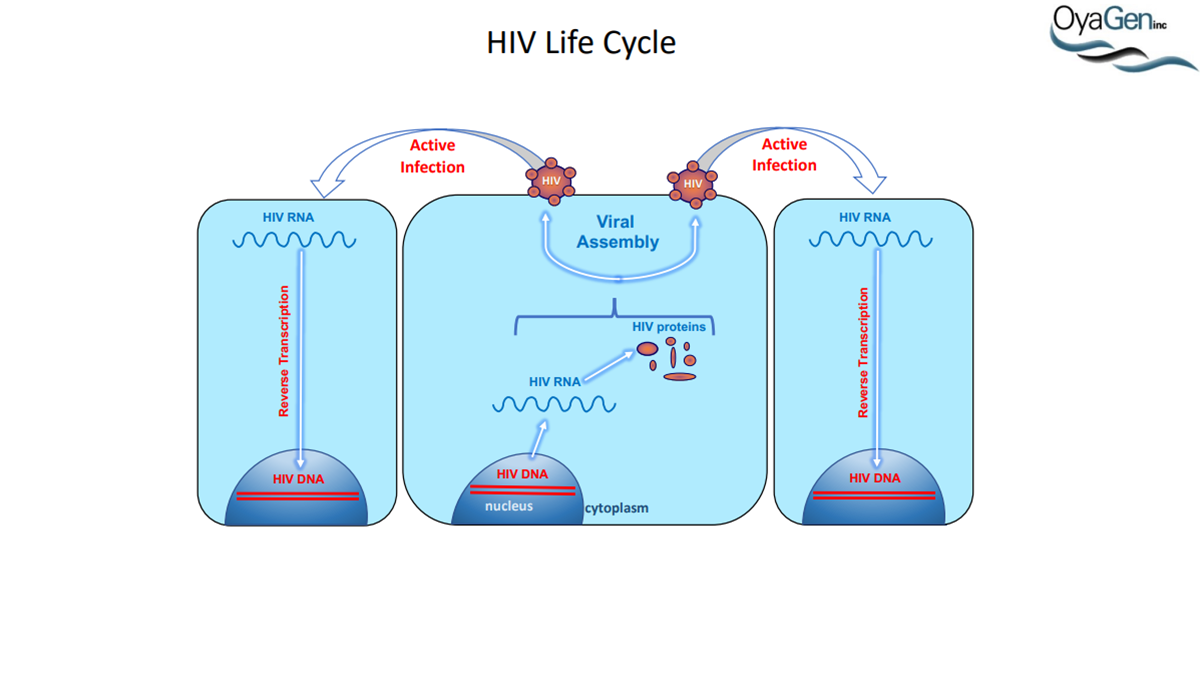
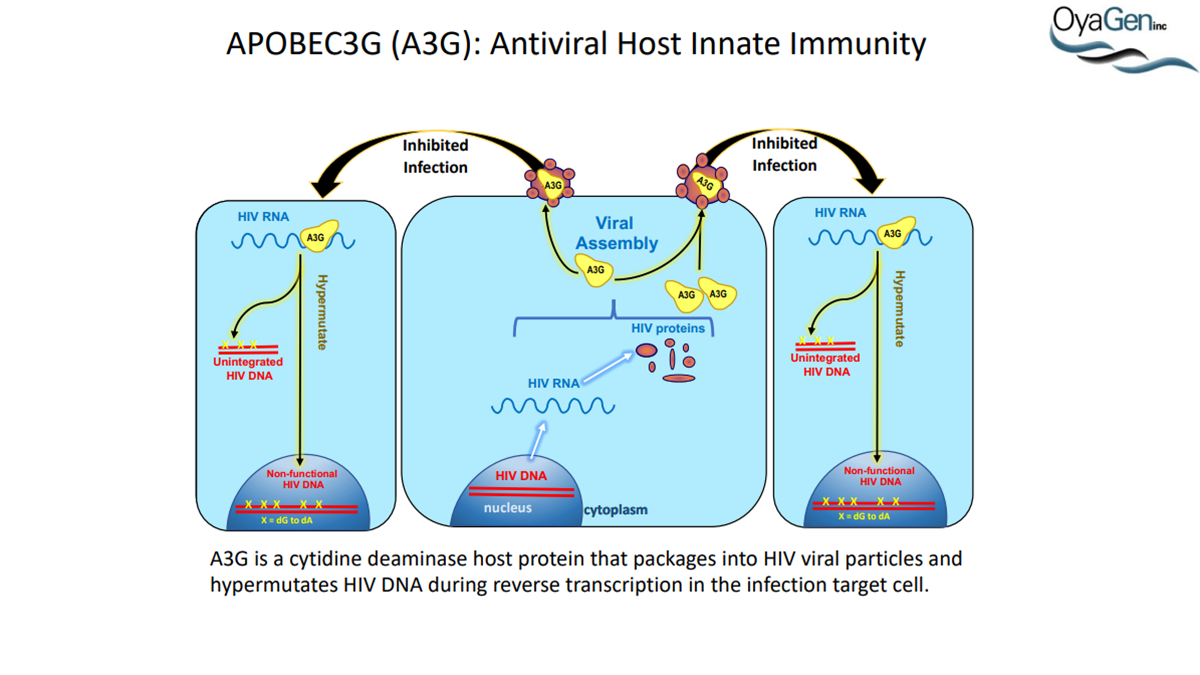
HIV-1 (HIV) is a lentivirus and the causative agent of AIDS. At the end of 2011, there were approximately 34 million people infected with HIV worldwide, with 2.5 million new infections. Infection rates are on the rise not only in under-developed countries but in high income nations as well. This is demonstrated by the fact that, in 2011, there were an estimated 1.4 million people infected in North America alone, which is a 22% increase from 2001 (http://www.unaids.org). Thus, one of the goals set by UNAIDS is to decrease sexual transmission of HIV by 50% by 2015, which will be aided by the development of novel prophylactic strategies. One potential mechanism for preventing HIV transmission is to activate a known host defense factor, APOBEC3G (A3G). Active A3G binds to viral single stranded DNA (ssDNA) and deaminates dC to dU, leading to hypermutated viral genomes and non-infectious virions. Targeting host proteins is often avoided due to the concern of toxic side effects. However, A3G is restricted to the cytoplasm [6], has no access to genomic DNA, and has no known cellular DNA substrates, making it a good therapeutic candidate. Activation of A3G enzymatic activity represents a novel preventative strategy and may be accomplished using a vaginal gel containing a small molecule A3G activator (SMAA). In addition, activating A3G in an infected individual holds the potential to be functionally curative as either a stand-alone treatment or in conjunction with additional treatment strategies.
In 1984, scientists at the US National Institutes of Health (NIH) and the Pasteur Institute determined that HIV was the virus that causes AIDS. At that time, it was declared that a vaccine would be available within two years. Nearly 30 years later, there remains no vaccine candidate and, in fact, NIH recently halted an HIV vaccine trial over concerns that more study participants became infected after receiving the test vaccine than those who received placebo. Thus, the only current hope for preventing HIV infection has come from Gilead’s combination treatment, StribildTM. This medication is a combination of 3 components of the current highly active anti-retroviral therapy (HAART) regiment (elvitegravir, an integrase inhibitor; emitracitabine and tenofivir, both nucleoside reverse transcriptase inhibitors) along with cobicistat (a pharmacokinetic enhancer of elvitegravir) (http://aidsinfo.nih.gov/guidelines). However, resistance to all three of these inhibitors had already emerged prior to combining and using them as a preventative (http://www.aidsmap.com), suggesting the same is likely to happen while using them to prevent HIV infection. In addition, if an at-risk individual is exposed to a strain resistant to any or all of the StribildTM components, there will be little chance for protection.
Not only has HIV developed resistance to the drugs currently in use as prophylactics, but also it has developed resistance to all HAART medications to date, including inhibitors of reverse transcriptase (RT; nucleoside and non-nucleoside), protease (PR), integrase (IN), and viral entry. The barrier to developing resistance to HIV drugs is low and, often times, a single codon change in the targeted protein is sufficient to cause resistance to more than one inhibitor of the same class (i.e. M46I/L/V in the PR enzyme confers resistance to 7 out of 8 inhibitors; http://www.aidsmap.com). To thoroughly emphasize this problem, ViiV Healthcare currently has a new IN inhibitor (dolutegravir) in Phase III clinical trials and in vitro passage experiments demonstrated the emergence of resistant viral strains after only 14 days. The ever-present problem of drug resistance together with the lack of success in developing an HIV vaccine underscore the need for novel prevention and treatment strategies that are unlikely to develop resistance, such as targeting the A3G host defense factor.
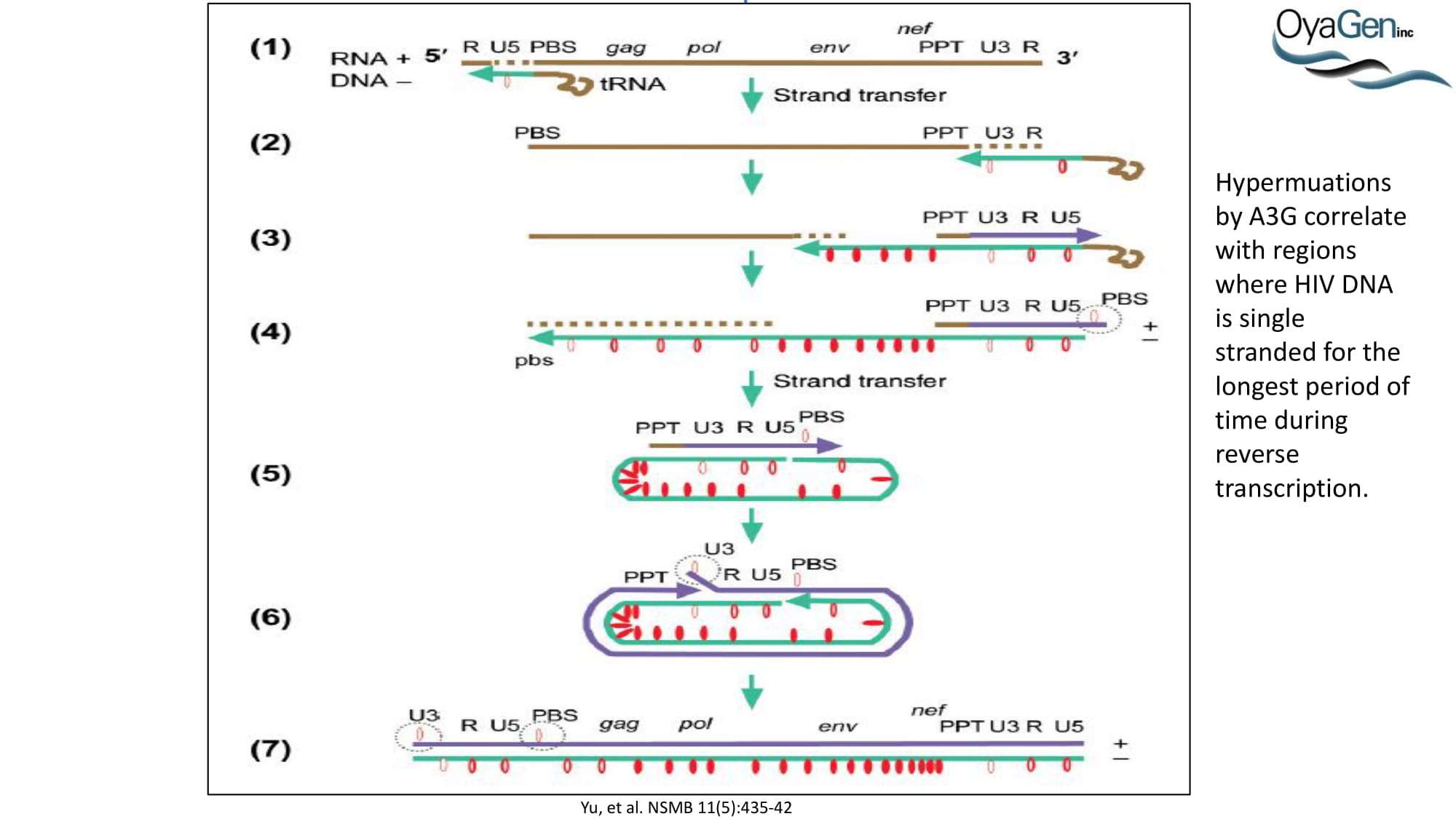
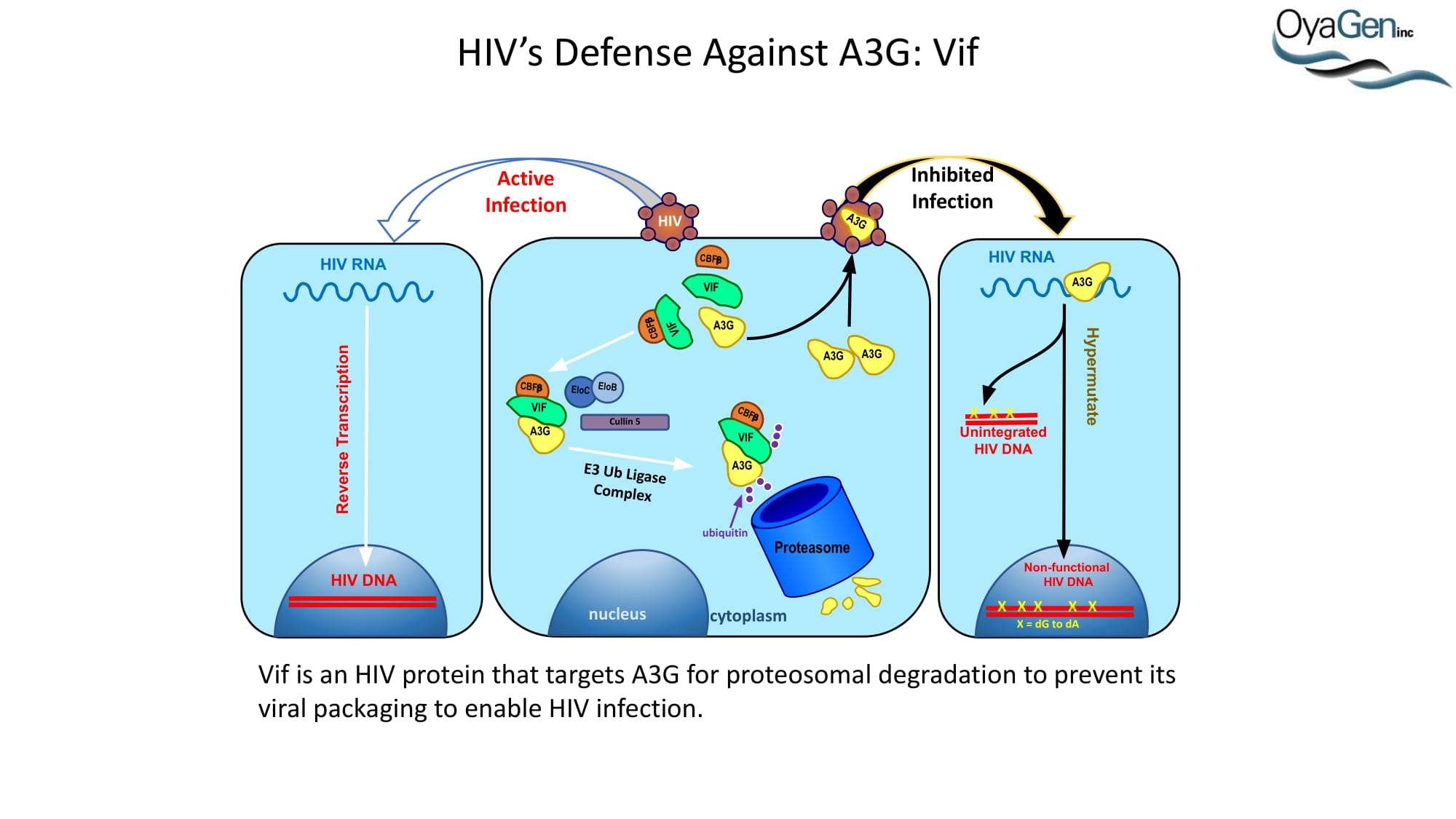
A3G was discovered as an HIV restriction factor during studies of the HIV Vif (viral infectivity factor) protein. Vif is a 23 kDa phosphoprotein that is expressed late in the viral life cycle. In the absence of Vif, HIV cannot establish a spreading infection in non-permissive cells (i.e. CD4 cells and macrophages). However, Vif expression is not required for infection of permissive cells (i.e. 293T, HeLa, and COS cells). Heterokaryons formed between permissive and non-permissive cells were infected with Vif-deficient virus and the resulting progeny virions were determined to be non-infectious, demonstrating that cells expressed an anti-HIV factor that was overcome by Vif expression. Subtractive hybridization experiments by Sheehy, et al identified A3G as the antiviral factor. A3G is a member of the cytidine deaminase family, all of which demonstrate either DNA or RNA editing activity. This was the first indication as to how A3G was functioning as an antiviral. Further studies determined that, in the absence of Vif, A3G is incorporated into HIV virions and, once encapsidated, A3G binds to the viral core. During viral replication, A3G extensively deaminates viral minus-strand DNA, converting dC to dU. This hypermutation of the viral DNA leads to one of two outcomes: 1) viral DNA is destroyed by DNA repair enzymes or 2) the mutated minus-strand DNA serves as a template for plus-strand synthesis whereby aberrant dU residues lead to dG to dA transitions. This alters the viral open reading frame, leading to the introduction of premature stop codons and missense codons. Each situation leads to the generation of less infectious virions and, thus, a decrease in HIV infectivity

© 2025, OyaGen, Inc.
SMARTSite by Site Hub