- Therapeutic Solutions
-
-
-
Therapeutic Solution
Ebola
COVID-19
Oncology
-
-
-
- Medical Animations
- Commercial Services
- Company Infrastructure
- People
- News
- Invest
- Contact
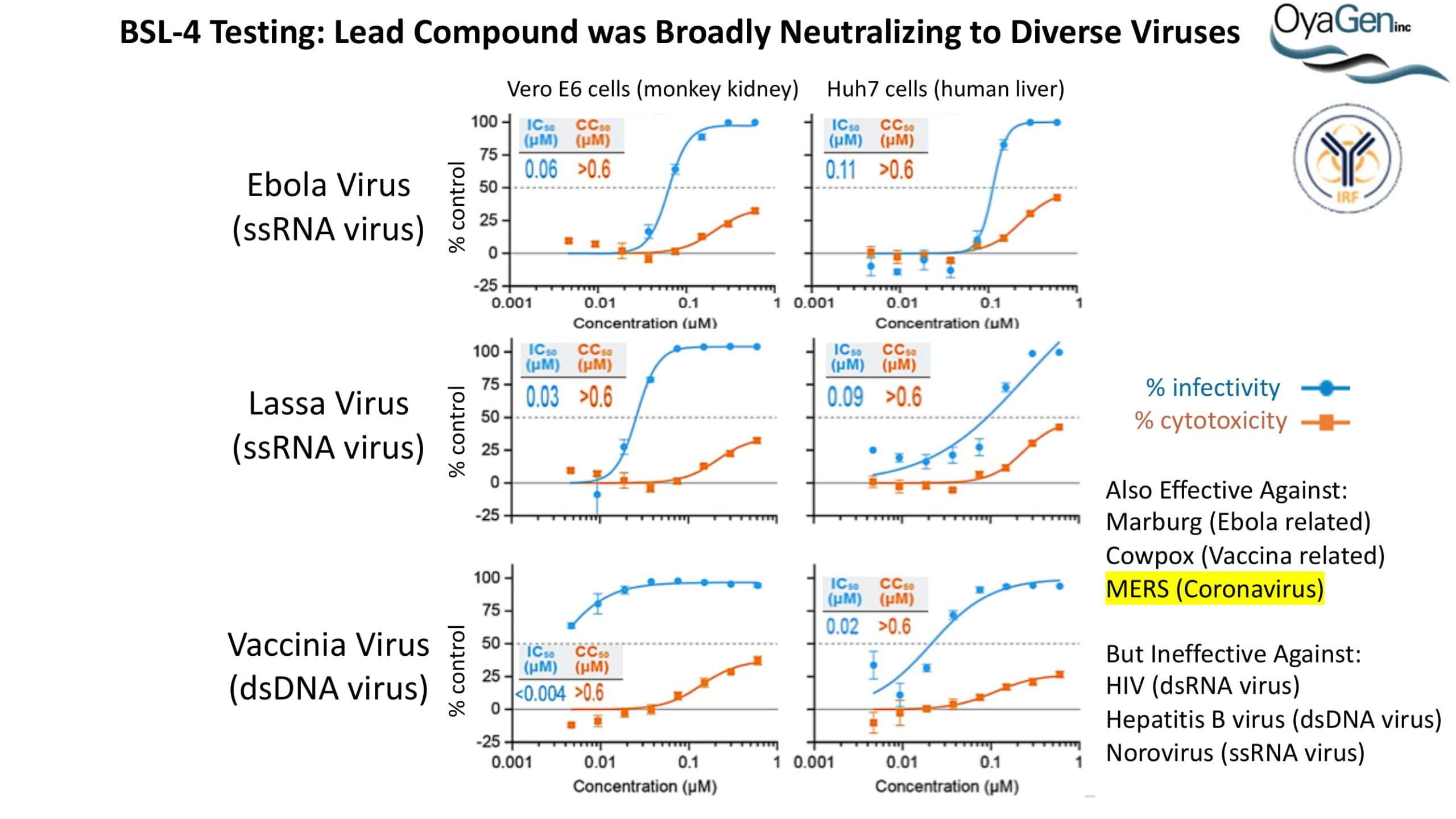
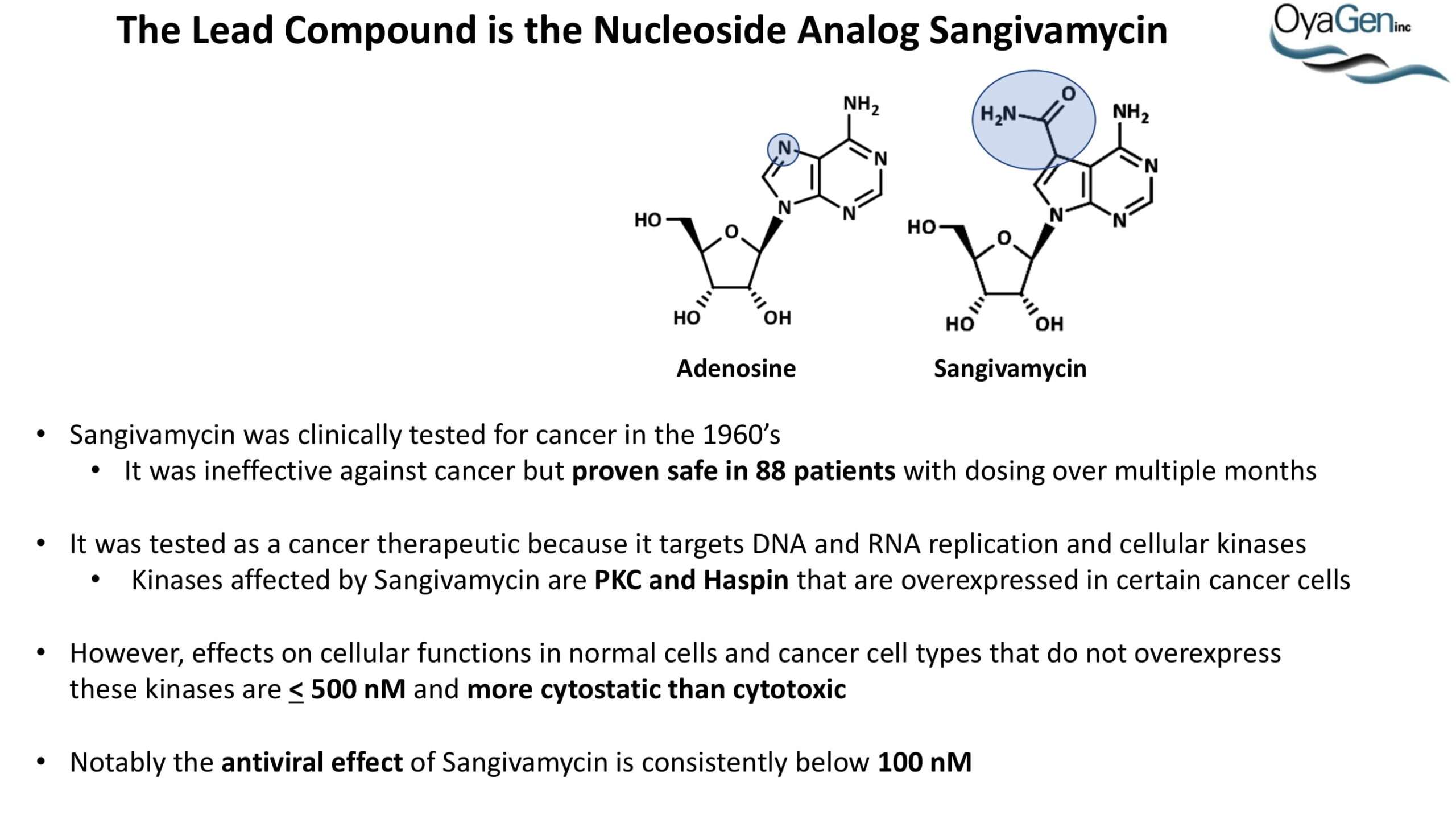
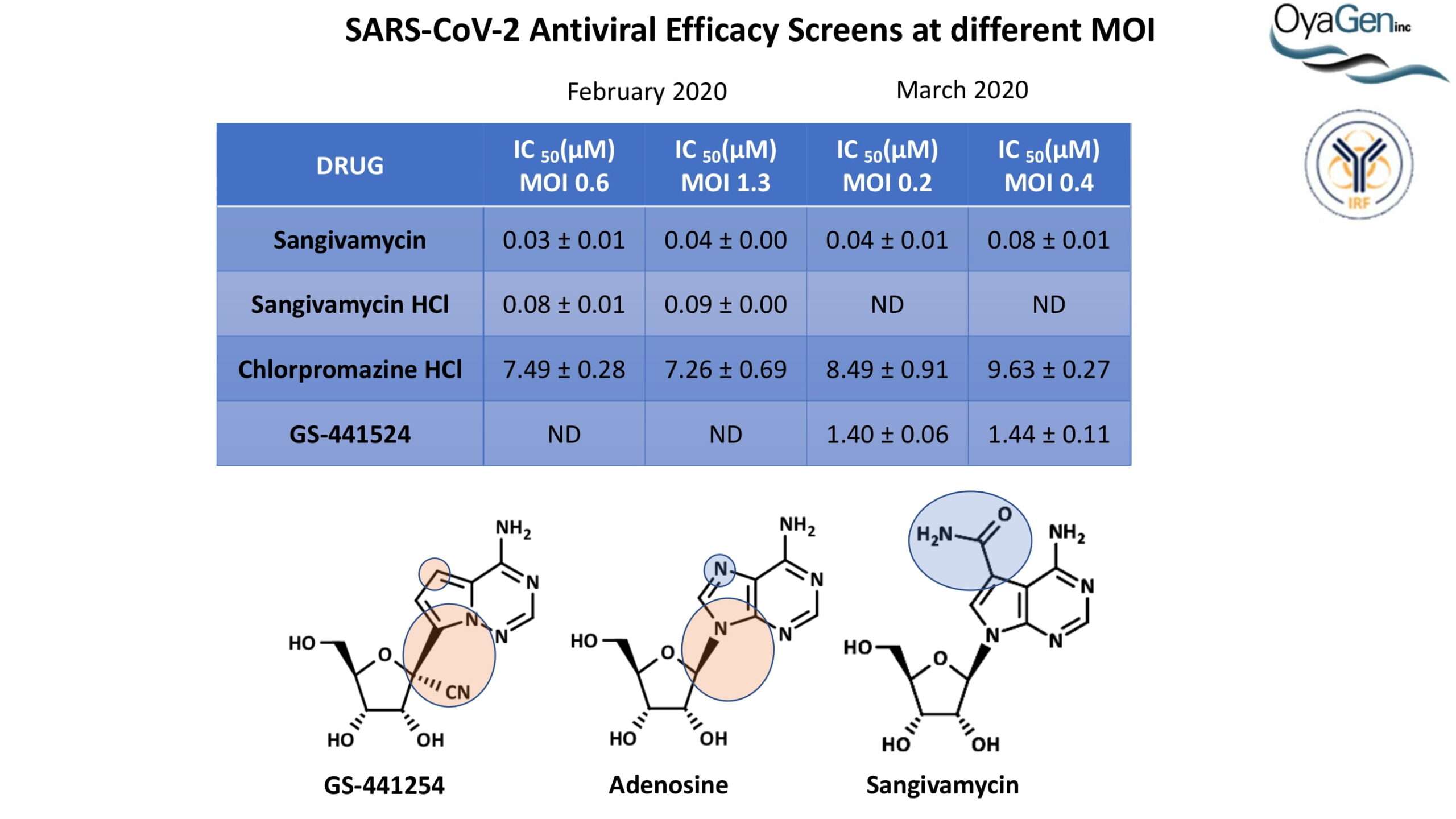
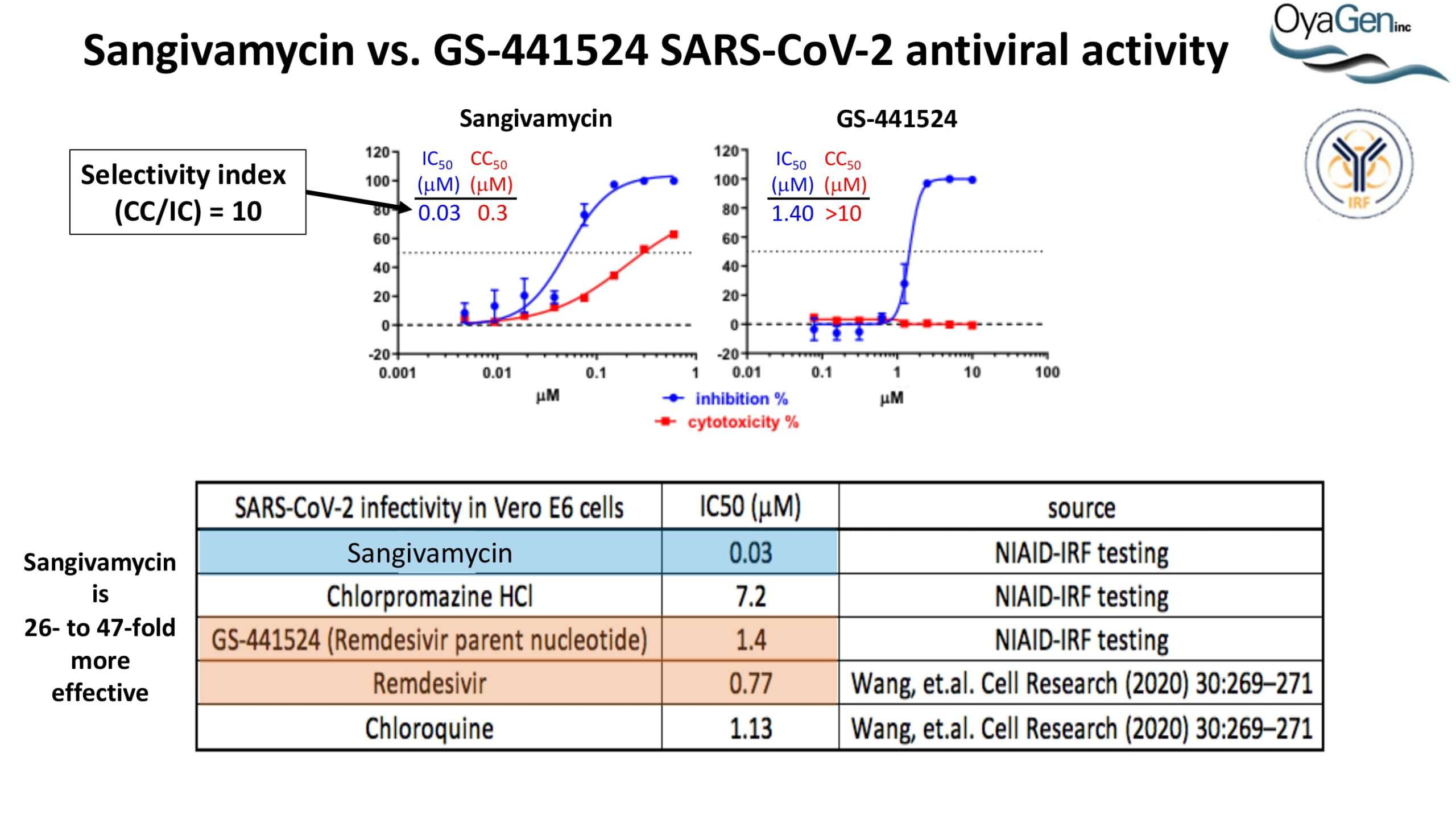
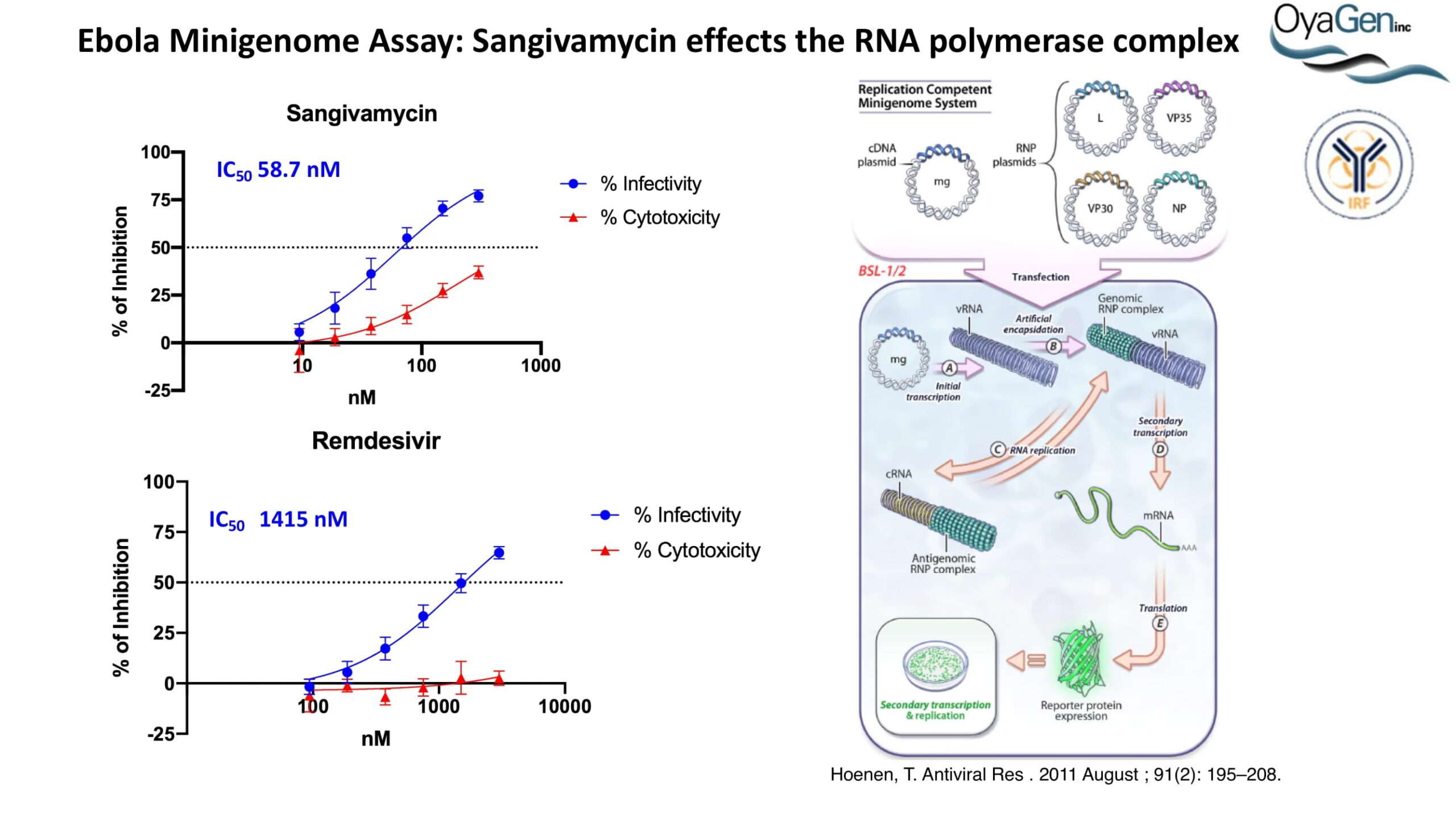
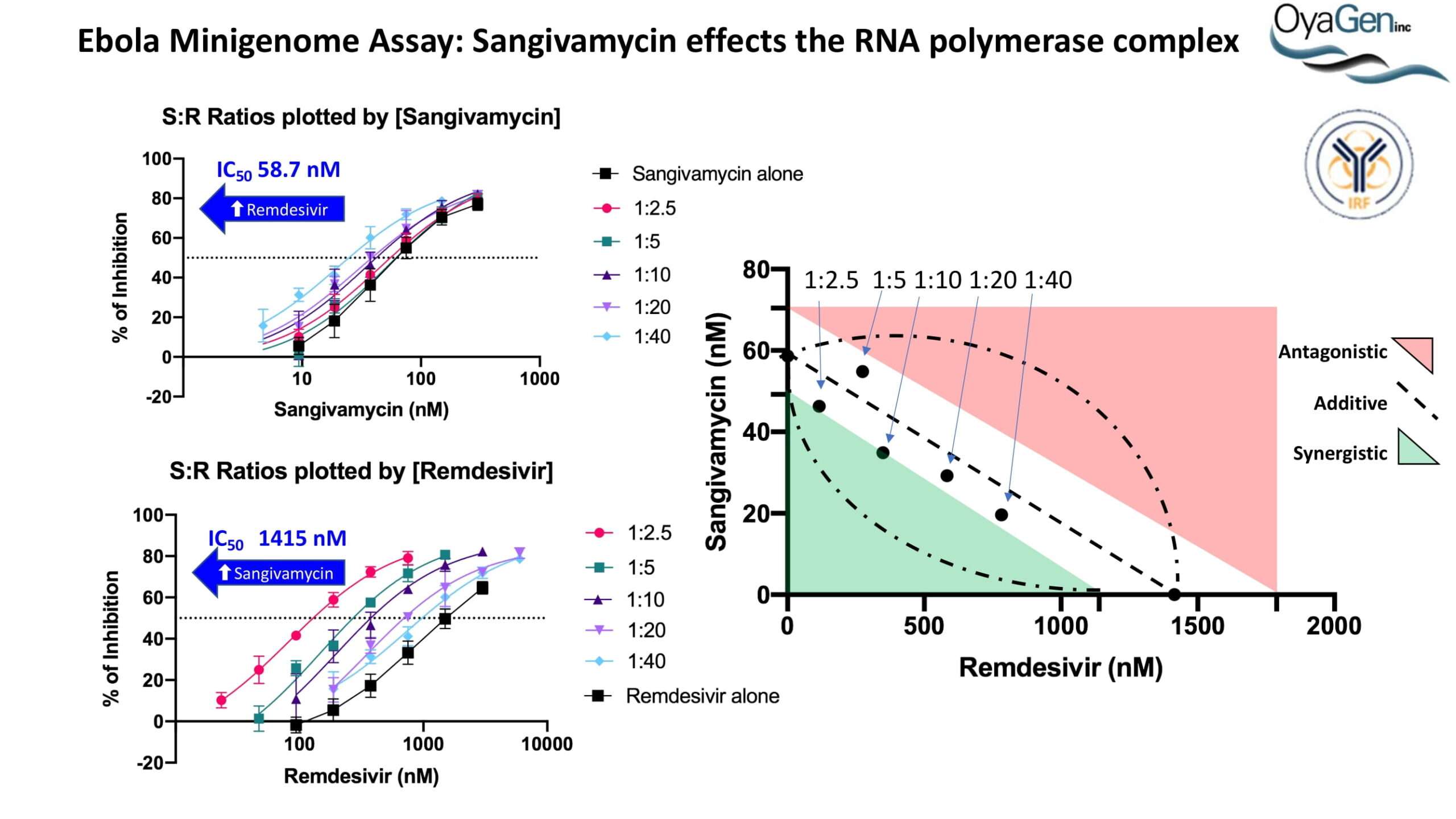
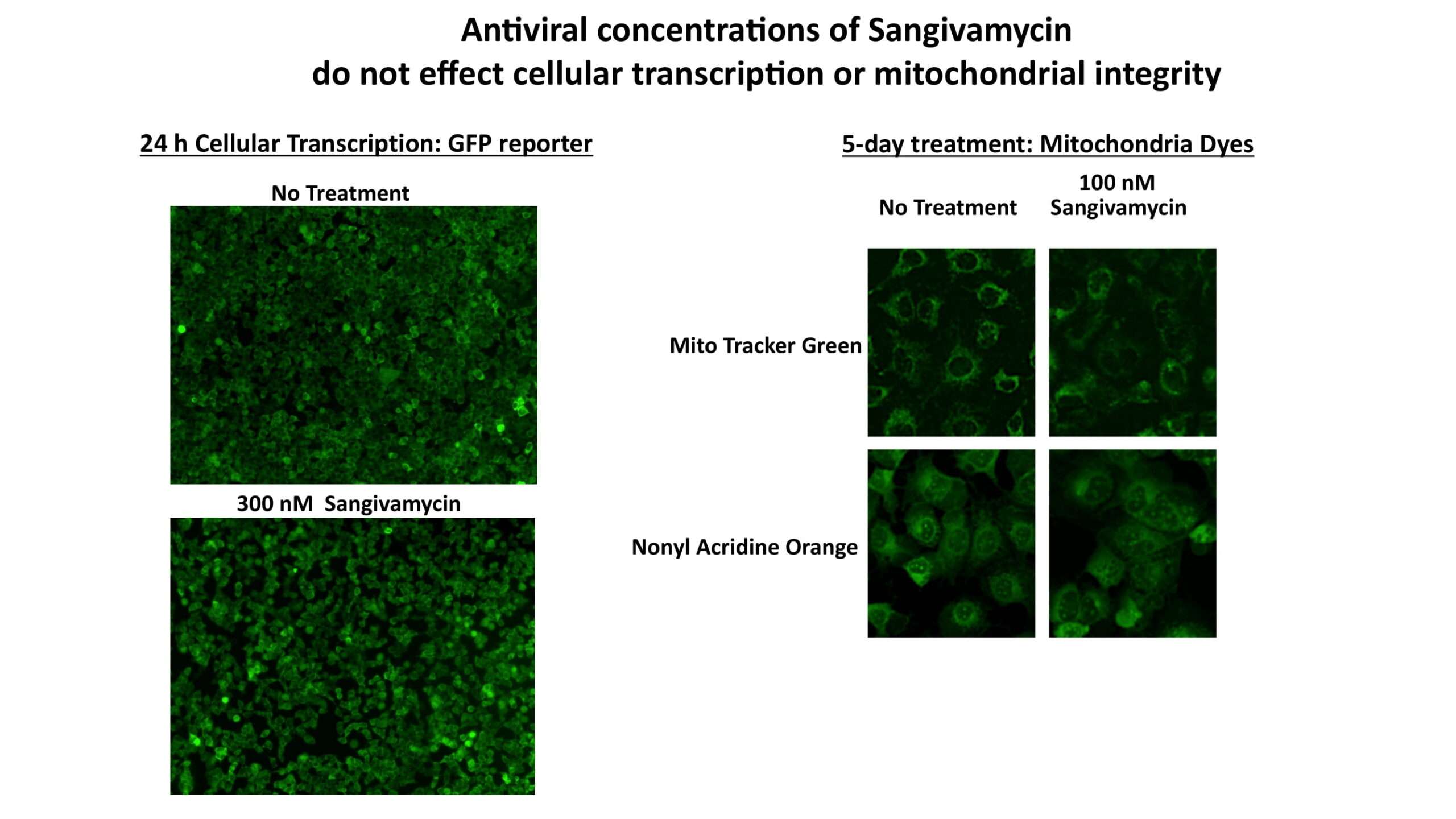
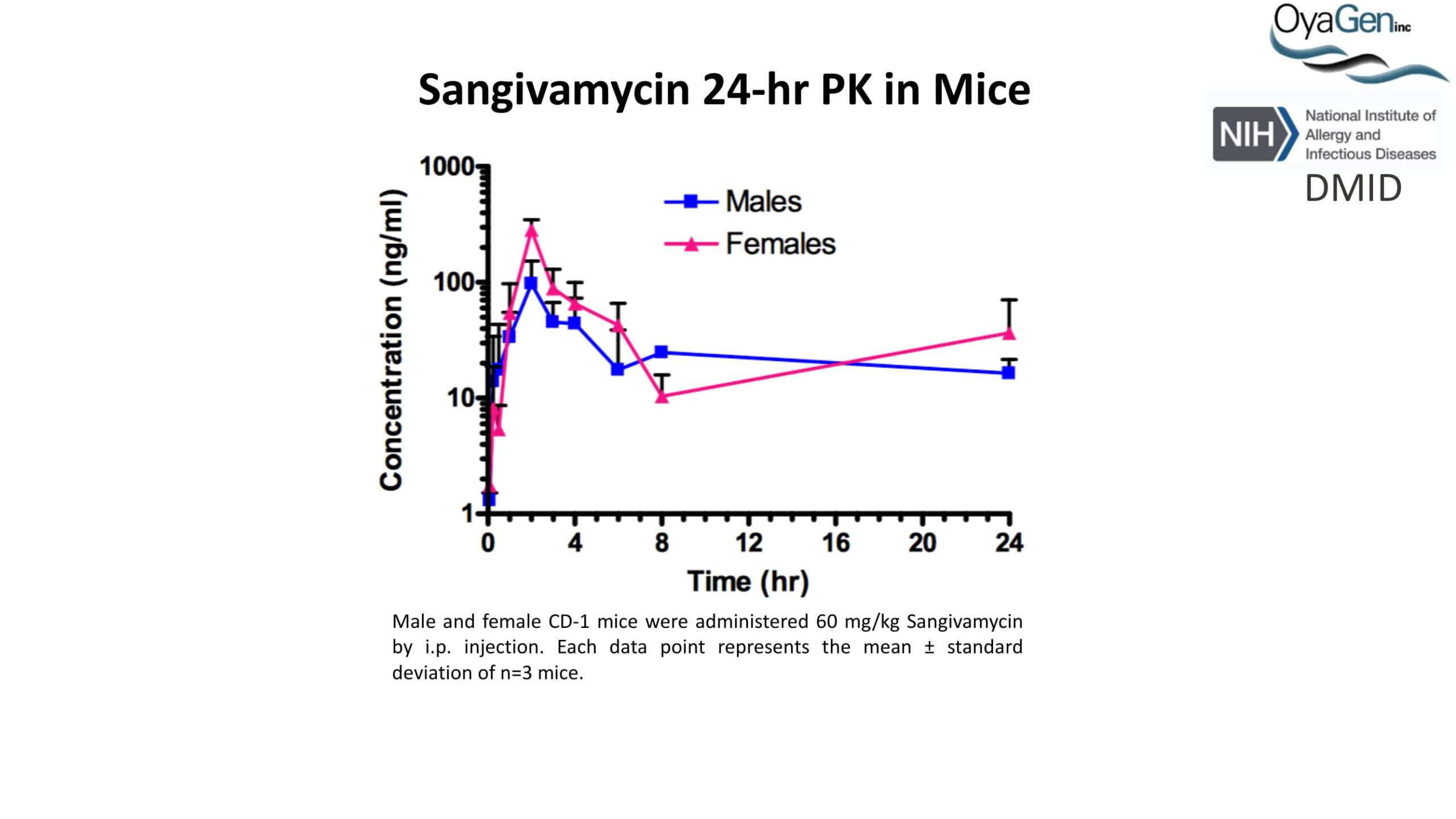
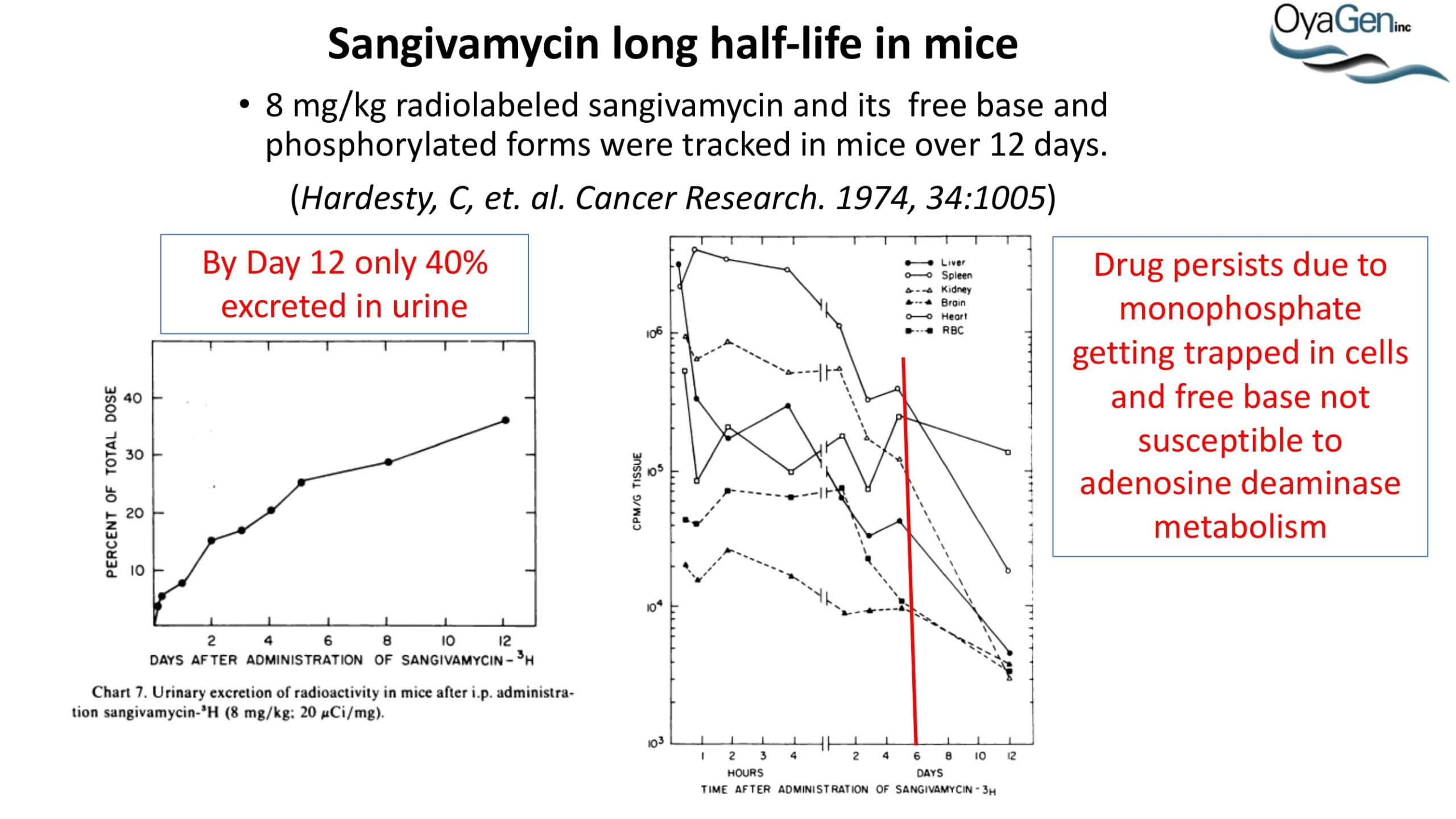
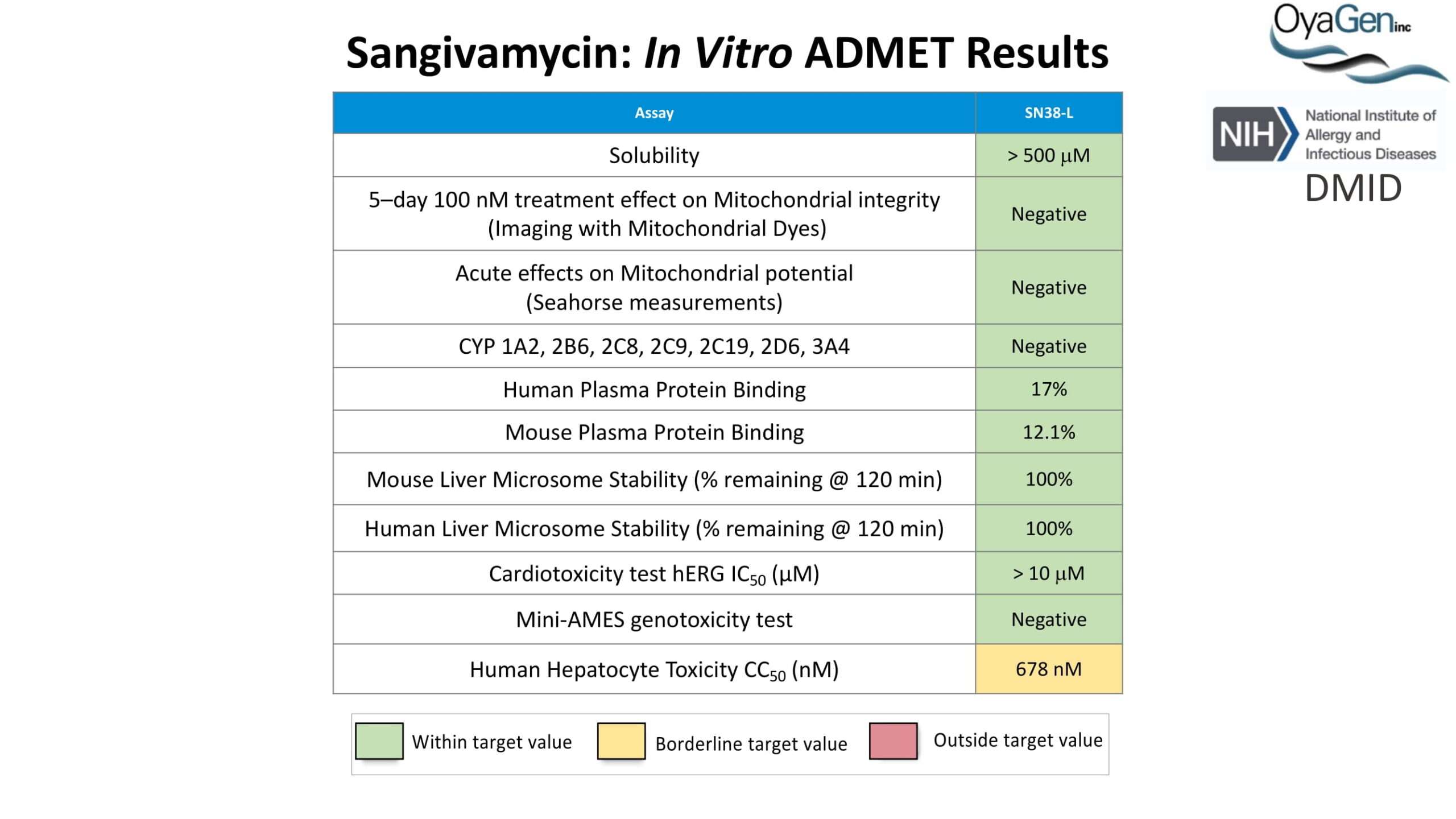
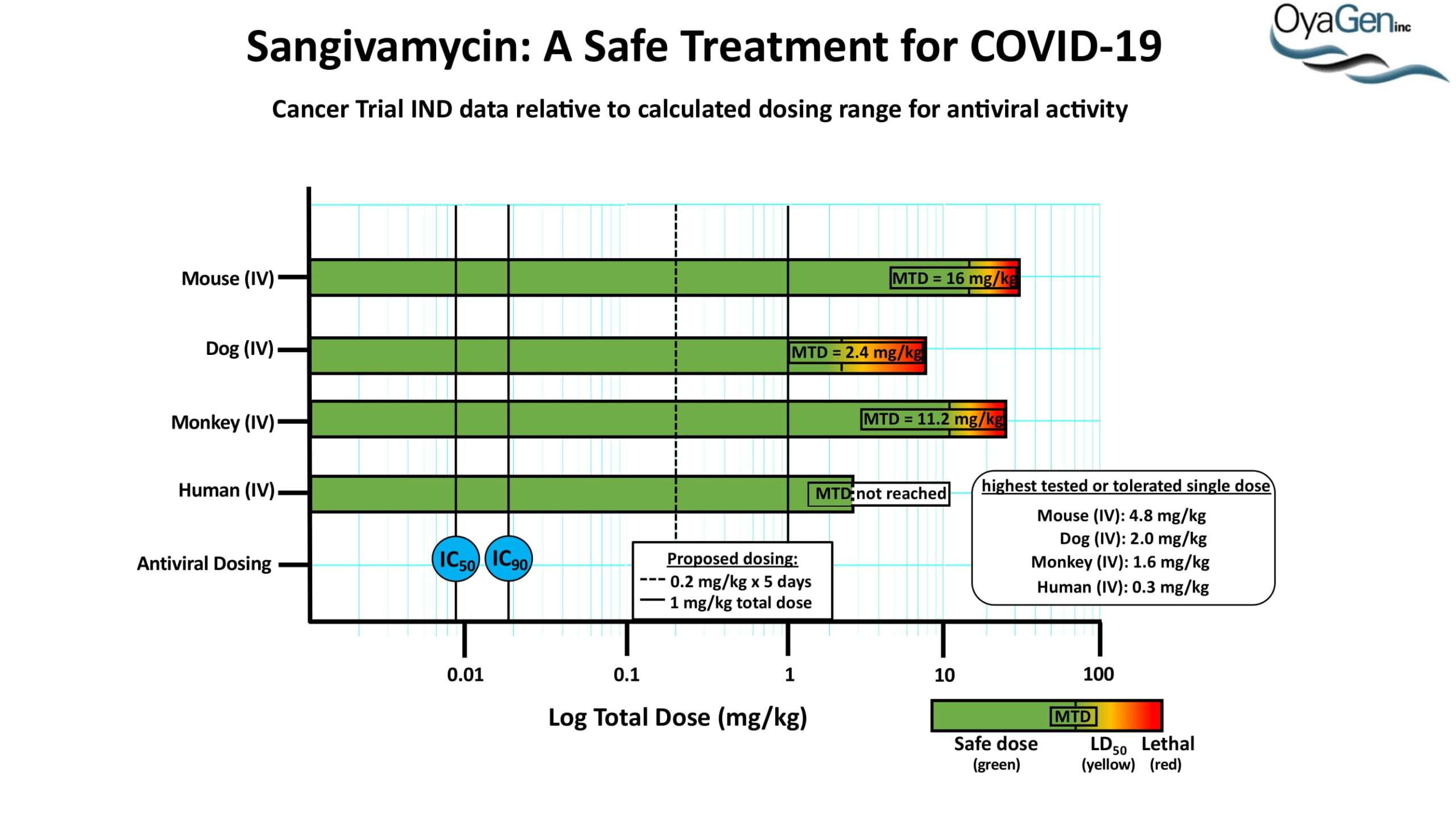
OyaGen developed Oya1 (aka sangivamycin) in collaboration with NIH/NIAID as a highly potent compound that prevents the spread of infection of Coronaviruses, Ebola, Lassa virus and Pox virus. Specifically, OyaGen has seven patents pending for this highly potent compound that prevents the spread of infection by Coronaviruses, Ebola, Lassa virus and Pox virus. The compound was taken through late-stage preclinical development by OyaGen. Oya1 has been safely administered to nonhuman primates and humans in the 1960’s during the war on cancer but was abandoned as being ineffective against cancer. Oya1 is a broad-spectrum antiviral and has excellent drug-like ADMET properties and higher potency relative to remdesivir in live virus studies. Oya1 antiviral activity is additive with remdesivir when the drugs are used in combination as shown in our publication in JCI Insight (10.1172/jci.insight.153165). Oya1 potential as a preventative for viral infections is suggested by its long half-life in tissues. Oya1 holds potential as a much needed, life-saving therapeutic approach for those who cannot benefit from a vaccine or will not take a vaccine.




© 2025, OyaGen, Inc.
SMARTSite by Site Hub